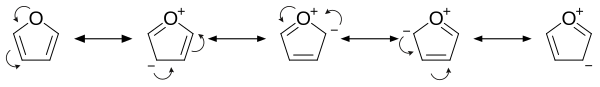J u m p t o c o n t e n t
M a i n m e n u
M a i n m e n u
N a v i g a t i o n
● M a i n p a g e ● C o n t e n t s ● C u r r e n t e v e n t s ● R a n d o m a r t i c l e ● A b o u t W i k i p e d i a ● C o n t a c t u s ● D o n a t e
C o n t r i b u t e
● H e l p ● L e a r n t o e d i t ● C o m m u n i t y p o r t a l ● R e c e n t c h a n g e s ● U p l o a d f i l e
S e a r c h
Search
A p p e a r a n c e
● C r e a t e a c c o u n t ● L o g i n
P e r s o n a l t o o l s
● C r e a t e a c c o u n t ● L o g i n
P a g e s f o r l o g g e d o u t e d i t o r s l e a r n m o r e ● C o n t r i b u t i o n s ● T a l k
( T o p )
1 H i s t o r y
2 P r o d u c t i o n
T o g g l e P r o d u c t i o n s u b s e c t i o n
2 . 1 S y n t h e s i s o f f u r a n s
3 S t r u c t u r e a n d b o n d i n g
4 R e a c t i v i t y
5 S a f e t y
6 S e e a l s o
7 R e f e r e n c e s
8 E x t e r n a l l i n k s
T o g g l e t h e t a b l e o f c o n t e n t s
F u r a n
4 7 l a n g u a g e s
● A f r i k a a n s ● ا ل ع ر ب ي ة ● A z ə r b a y c a n c a ● ت ۆ ر ک ج ه ● Б е л а р у с к а я ● Б ъ л г а р с к и ● B o s a n s k i ● C a t a l à ● Č e š t i n a ● D e u t s c h ● E e s t i ● Ε λ λ η ν ι κ ά ● E s p a ñ o l ● E s p e r a n t o ● E u s k a r a ● ف ا ر س ی ● F r a n ç a i s ● G a l e g o ● 한 국 어 ● Հ ա յ ե ր ե ն ● B a h a s a I n d o n e s i a ● I t a l i a n o ● ქ ა რ თ უ ლ ი ● К ы р г ы з ч а ● L a t v i e š u ● M a g y a r ● М а к е д о н с к и ● N e d e r l a n d s ● 日 本 語 ● N o r s k b o k m å l ● O ʻ z b e k c h a / ў з б е к ч а ● P o l s k i ● P o r t u g u ê s ● R o m â n ă ● Р у с с к и й ● S l o v e n č i n a ● S l o v e n š č i n a ● С р п с к и / s r p s k i ● S r p s k o h r v a t s k i / с р п с к о х р в а т с к и ● S u o m i ● S v e n s k a ● த ம ி ழ ் ● T ü r k ç e ● У к р а ї н с ь к а ● T i ế n g V i ệ t ● 吴 语 ● 中 文
E d i t l i n k s
● A r t i c l e ● T a l k
E n g l i s h
● R e a d ● E d i t ● V i e w h i s t o r y
T o o l s
T o o l s
A c t i o n s
● R e a d ● E d i t ● V i e w h i s t o r y
G e n e r a l
● W h a t l i n k s h e r e ● R e l a t e d c h a n g e s ● U p l o a d f i l e ● S p e c i a l p a g e s ● P e r m a n e n t l i n k ● P a g e i n f o r m a t i o n ● C i t e t h i s p a g e ● G e t s h o r t e n e d U R L ● D o w n l o a d Q R c o d e ● W i k i d a t a i t e m
P r i n t / e x p o r t
● D o w n l o a d a s P D F ● P r i n t a b l e v e r s i o n
I n o t h e r p r o j e c t s
● W i k i m e d i a C o m m o n s
A p p e a r a n c e
F r o m W i k i p e d i a , t h e f r e e e n c y c l o p e d i a
Heterocyclic organic compound,
Furan
Full structural formula of furan Skeletal formula showing numbering convention
Ball-and-stick model Space-filling model
Names
Preferred IUPAC name
Systematic IUPAC name
1,4-Epoxybuta-1,3-diene
Other names
Oxole5 ]annulene
Identifiers
CAS Number
3D model (JSmol )
Beilstein Reference
103221
ChEBI
ChEMBL
ChemSpider
ECHA InfoCard 100.003.390
EC Number
Gmelin Reference
25716
KEGG
PubChem CID
RTECS number
UNII
UN number
2389
CompTox Dashboard (EPA )
InChI=1S/C4H4O/c1-2-4-5-3-1/h1-4H Y
Key: YLQBMQCUIZJEEH-UHFFFAOYSA-N Y
InChI=1/C4H4O/c1-2-4-5-3-1/h1-4H
Key: YLQBMQCUIZJEEH-UHFFFAOYAC
Properties
Chemical formula
C 4 H 4 O
Molar mass
−1
Appearance
Colorless, volatile liquid
Density
0.936 g/mL
Melting point
−85.6 °C (−122.1 °F; 187.6 K )
Boiling point
31.3 °C (88.3 °F; 304.4 K )
Magnetic susceptibility (χ)
-43.09·10−6 cm 3
Hazards
GHS labelling
Pictograms
Signal word
Danger
Hazard statements
H224 , H302 , H315 , H332 , H341 , H350 , H373 , H412
Precautionary statements
P201 , P202 , P210 , P233 , P240 , P241 , P242 , P243 , P260 , P261 , P264 , P270 , P271 , P273 , P280 , P281 , P301+P312 , P302+P352 , P303+P361+P353 , P304+P312 , P304+P340 , P308+P313 , P312 , P314 , P321 , P330 , P332+P313 , P362 , P370+P378 , P403+P235 , P405 , P501
NFPA 704
Flash point
−36 °C (−33 °F; 237 K )
Autoignition
390 °C (734 °F; 663 K )
Explosive limits
Lower: 2.3%
Lethal dose or concentration (LD, LC):
LD 50 median dose )
> 2 g/kg (rat)
Safety data sheet (SDS)
Pennakem
Related compounds
Related heterocycles
Pyrrole Thiophene
Related compounds
Tetrahydrofuran (THF)2,5-Dimethylfuran Benzofuran Dibenzofuran
Structure
Point group
C 2v
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
Chemical compound
Furan is a heterocyclic organic compound , consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.
Furan is a colorless, flammable , highly volatile liquid with a boiling point close to room temperature. It is soluble in common organic solvents , including alcohol , ether , and acetone , and is slightly soluble in water .[2] chloroform -like".[3] toxic and may be carcinogenic in humans. Furan is used as a starting point for other speciality chemicals .[4]
History [ edit ]
The name "furan" comes from the Latin furfur , which means bran [5] furfural is produced from bran). The first furan derivative to be described was 2-furoic acid , by Carl Wilhelm Scheele in 1780. Another important derivative, furfural , was reported by Johann Wolfgang Döbereiner in 1831 and characterised nine years later by John Stenhouse . Furan itself was first prepared by Heinrich Limpricht in 1870, although he called it "tetraphenol" (as if it were a four-carbon analog to phenol , C6 H 5 OH ).[6] [7]
Production [ edit ]
Industrially, furan is manufactured by the palladium -catalyzed decarbonylation of furfural , or by the copper -catalyzed oxidation of 1,3-butadiene :[4]
In the laboratory, furan can be obtained from furfural by oxidation to 2-furoic acid, followed by decarboxylation .[8] thermal decomposition of pentose -containing materials, and cellulosic solids, especially pine wood.
Synthesis of furans [ edit ]
The Feist–Benary synthesis is a classic way to synthesize furans. The reaction involves alkylation of 1,3-diketones with α-bromoketones followed by dehydration of an intermediate hydroxydihydrofuran .[9] 1,4-diketones with phosphorus pentoxide (P 2 O 5 Paal–Knorr synthesis .[10]
Many routes exist for the synthesis of substituted furans.[11] [12]
Furan in nature and commerce
The drug Zantac, also known as
ranitidine .
Rosefuran , an aroma compound found in rose oil.
[10]
Furfural , derived from sugars, is the major source of furans
Structure and bonding [ edit ]
Furan has aromatic character because one of the lone pairs of electrons on the oxygen atom is delocalized into the ring, creating a 4n Hückel's rule ). The aromaticity is modest relative to that for benzene and related heterocycles thiophene and pyrrole . The resonance energies of benzene, pyrrole , thiophene , and furan are, respectively, 152, 88, 121, and 67 kJ/mol (36, 21, 29, and 16 kcal/mol). Thus, these heterocycles, especially furan, are far less aromatic than benzene, as is manifested in the lability of these rings.[13] double bond character. The other lone pair of electrons of the oxygen atom extends in the plane of the flat ring system.
Examination of the resonance contributors shows the increased electron density of the ring, leading to increased rates of electrophilic substitution.[14]
Reactivity [ edit ]
Because of its partial aromatic character, furan's behavior is intermediate between that of an enol ether and an aromatic ring. It is dissimilar vs ethers such as tetrahydrofuran .
Like enol ethers, 2,5-disubstituted furans are susceptible to hydrolysis to reversibly give 1,4-diketones.
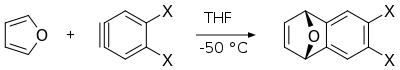
Furan serves as a diene in Diels–Alder reactions with electron-deficient dienophiles such as ethyl (E [15] endo isomer
Diels-Alder reaction of furan with arynes provides corresponding derivatives of dihydronaphthalenes , which are useful intermediates in synthesis of other polycyclic aromatic compounds .[16]
It is considerably more reactive than benzene in electrophilic substitution reactions, due to the electron-donating effects of the oxygen heteroatom. It reacts with bromine at 0 °C to give 2-bromofuran.
Furan is found in heat-treated commercial foods and is produced through thermal degradation of natural food constituents.[18] [19] coffee , instant coffee, and processed baby foods .[19] [20] [21] espresso makers and coffee made from capsules contain more furan than that made in traditional drip coffee makers , although the levels are still within safe health limits.[22]
Exposure to furan at doses about 2,000 times the projected level of human exposure from foods increases the risk of hepatocellular tumors in rats and mice and bile duct tumors in rats.[23] carcinogen .[23]
See also [ edit ]
References [ edit ]
^ Jakubke, Hans Dieter; Jeschkeit, Hans (1994). Concise Encyclopedia of Chemistry 1–1201 . ISBN 0-89925-457-8
^ DHHS (NIOSH) Publication No. 2016–171 , p. 2, Accessed Nov 2019
^ a b Hoydonckx, H. E.; Van Rhijn, W. M.; Van Rhijn, W.; De Vos, D. E.; Jacobs, P. A. "Furfural and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry doi :10.1002/14356007.a12_119.pub2 . ISBN 978-3527306732
^ Senning, Alexander (2006). Elsevier's Dictionary of Chemoetymology . Elsevier. ISBN 0-444-52239-5
^ Limpricht, H. (1870). "Ueber das Tetraphenol C4 H 4 . Berichte der Deutschen Chemischen Gesellschaft . 3 1 ): 90–91. doi :10.1002/cber.18700030129 .
^ Rodd, Ernest Harry (1971). Chemistry of Carbon Compounds: A Modern Comprehensive Treatise . Elsevier.
^ Wilson, W. C. (1941). "Furan" . Organic Syntheses Collected Volumes , vol. 1, p. 274
^ Hou, X. L.; Cheung, H. Y.; Hon, T. Y.; Kwan, P. L.; Lo, T. H.; Tong, S. Y.; Wong, H. N. (1998). "Regioselective syntheses of substituted furans". Tetrahedron 54 10 ): 1955–2020. doi :10.1016/S0040-4020(97 )10303-9 .
^ a b Gilchrist, Thomas L. (1997). Heterocyclic Chemistry (3rd ed.). Liverpool: Longman. p. 209-212.
^ Adam Sniady, Marco S. Morreale, Roman Dembinski (2007). "Electrophilic Cyclization with N-Iodosuccinimide: Preparation of 5-(4-Bromophenyl)-3-Iodo-2-(4-Methyl-Phenyl)Furan". Organic Syntheses . 84 doi :10.15227/orgsyn.084.0199 . {{cite journal }}: CS1 maint: multiple names: authors list (link )
^ James A. Marshall, Clark A. Sehon (1999). "Isomerization of b-Alkynyl Allylic Alcohols to Furans Catalyzed by Silver Nitrate on Silica Gel: 2-Pentyl-3-Methyl-5-Heptylfuran". Organic Syntheses . 76 doi :10.15227/orgsyn.076.0263 .
^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure ISBN 978-0-471-72091-1
^ Bruice, Paula Y. (2007). Organic Chemistry (5th ed.). Upper Saddle River, NJ: Pearson Prentice Hall. ISBN 978-0-13-196316-0
^ Masesane, I.; Batsanov, A.; Howard, J.; Modal, R.; Steel, P. (2006). "The oxanorbornene approach to 3-hydroxy, 3,4-dihydroxy and 3,4,5-trihydroxy derivatives of 2-aminocyclohexanecarboxylic acid" . Beilstein Journal of Organic Chemistry 2 9 ): 9. doi :10.1186/1860-5397-2-9 PMC 1524792 PMID 16674802 .
^ Filatov, M. A.; Baluschev, S.; Ilieva, I. Z.; Enkelmann, V.; Miteva, T.; Landfester, K. ; Aleshchenkov, S. E.; Cheprakov, A. V. (2012). "Tetraaryltetraanthra[2,3]porphyrins: Synthesis, Structure, and Optical Properties" (PDF) . J. Org. Chem . 77 24 ): 11119–11131. doi :10.1021/jo302135q . PMID 23205621 . Archived from the original (PDF) on 2020-02-19.
^ Harreus, Albrecht Ludwig. "Pyrrole". Ullmann's Encyclopedia of Industrial Chemistry doi :10.1002/14356007.a22_453 . ISBN 978-3527306732
^ Anese, M.; Manzocco, L.; Calligaris, S.; Nicoli, M. C. (2013). "Industrially Applicable Strategies for Mitigating Acrylamide, Furan and 5-Hydroxymethylfurfural in Food" (PDF) . Journal of Agricultural and Food Chemistry . 61 43 ): 10209–14. doi :10.1021/jf305085r . PMID 23627283 . Archived from the original (PDF) on 2017-08-08.
^ a b Moro, S.; Chipman, J. K.; Wegener, J. W.; Hamberger, C.; Dekant, W.; Mally, A. (2012). "Furan in heat-treated foods: Formation, exposure, toxicity, and aspects of risk assessment" (PDF) . Molecular Nutrition & Food Research . 56 8 ): 1197–1211. doi :10.1002/mnfr.201200093 . hdl :1871/41889 . PMID 22641279 . S2CID 12446132 .
^ European Food Safety Authority (2011). "Update on furan levels in food from monitoring years 2004–2010 and exposure assessment" . EFSA Journal . 9 9 ): 2347. doi :10.2903/j.efsa.2011.2347
^ Waizenegger, J.; Winkler, G.; Kuballa, T.; Ruge, W.; Kersting, M.; Alexy, U.; Lachenmeier, D. W. (2012). "Analysis and risk assessment of furan in coffee products targeted to adolescents". Food Additives & Contaminants: Part A . 29 1 ): 19–28. doi :10.1080/19440049.2011.617012 . PMID 22035212 . S2CID 29027966 .
^ "Espresso makers: Coffee in capsules contains more furan than the rest" . Science Daily
^ a b Bakhiya, N.; Appel, K. E. (2010). "Toxicity and carcinogenicity of furan in human diet" (PDF) . Archives of Toxicology . 84 7 ): 563–578. doi :10.1007/s00204-010-0531-y . PMID 20237914 . S2CID 19389984 .
External links [ edit ]
Wikimedia Commons has media related to
Furan .
t
e
1 ring
Three-membered
Five-membered
Six-membered
Seven-membered
Nine-membered
18-membered
2 rings
Five + Five
Five + Six
Six + Six
Five + Seven
R e t r i e v e d f r o m " https://en.wikipedia.org/w/index.php?title=Furan&oldid=1228320463 " C a t e g o r i e s : ● F u r a n s ● I A R C G r o u p 2 B c a r c i n o g e n s ● S i m p l e a r o m a t i c r i n g s ● S u b s t a n c e s d i s c o v e r e d i n t h e 1 9 t h c e n t u r y H i d d e n c a t e g o r i e s : ● C S 1 m a i n t : m u l t i p l e n a m e s : a u t h o r s l i s t ● A r t i c l e s w i t h s h o r t d e s c r i p t i o n ● S h o r t d e s c r i p t i o n i s d i f f e r e n t f r o m W i k i d a t a ● E C H A I n f o C a r d I D f r o m W i k i d a t a ● C h e m b o x h a v i n g G H S d a t a ● A r t i c l e s c o n t a i n i n g u n v e r i f i e d c h e m i c a l i n f o b o x e s ● S h o r t d e s c r i p t i o n m a t c h e s W i k i d a t a ● A l l a r t i c l e s w i t h u n s o u r c e d s t a t e m e n t s ● A r t i c l e s w i t h u n s o u r c e d s t a t e m e n t s f r o m S e p t e m b e r 2 0 2 3 ● C o m m o n s c a t e g o r y l i n k i s o n W i k i d a t a ● A r t i c l e s w i t h B N F i d e n t i f i e r s ● A r t i c l e s w i t h B N F d a t a i d e n t i f i e r s ● A r t i c l e s w i t h G N D i d e n t i f i e r s ● A r t i c l e s w i t h J 9 U i d e n t i f i e r s ● A r t i c l e s w i t h L C C N i d e n t i f i e r s ● A r t i c l e s w i t h N D L i d e n t i f i e r s ● A r t i c l e s w i t h N K C i d e n t i f i e r s
● T h i s p a g e w a s l a s t e d i t e d o n 1 0 J u n e 2 0 2 4 , a t 1 6 : 0 8 ( U T C ) . ● T e x t i s a v a i l a b l e u n d e r t h e C r e a t i v e C o m m o n s A t t r i b u t i o n - S h a r e A l i k e L i c e n s e 4 . 0 ;
a d d i t i o n a l t e r m s m a y a p p l y . B y u s i n g t h i s s i t e , y o u a g r e e t o t h e T e r m s o f U s e a n d P r i v a c y P o l i c y . W i k i p e d i a ® i s a r e g i s t e r e d t r a d e m a r k o f t h e W i k i m e d i a F o u n d a t i o n , I n c . , a n o n - p r o f i t o r g a n i z a t i o n . ● P r i v a c y p o l i c y ● A b o u t W i k i p e d i a ● D i s c l a i m e r s ● C o n t a c t W i k i p e d i a ● C o d e o f C o n d u c t ● D e v e l o p e r s ● S t a t i s t i c s ● C o o k i e s t a t e m e n t ● M o b i l e v i e w