


Zerovalent iron (ZVI) is jargon that describes forms of iron metal that are proposed for used in Groundwater remediation.[1][2][3][4]
Click either image to enlarge
ZVI operates by electron transfer from Fe0 toward some organochlorine compounds, a common class of pollutants. The remediation process is proposed to generate Fe2+ and Cl− and halide-free organic products, all of which are relatively innocuous.[5] The technology is not however been implemented, despite many proofs of principle.
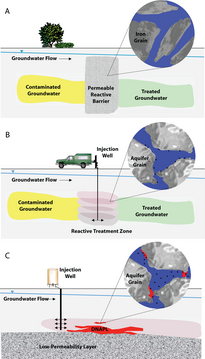
Many kinds of pollutants have been proposed, but few have been demonstrated in solving environmental challenges.
{{cite journal}}: CS1 maint: numeric names: authors list (link)