

| |
| Names | |
|---|---|
| Systematic IUPAC name
Nitrate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| NO− 3 | |
| Molar mass | 62.004 g·mol−1 |
| Conjugate acid | Nitric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
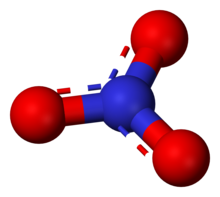
Nitrate is a polyatomic ion with the chemical formula NO−
3. Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives.[1] Almost all inorganic nitrates are solubleinwater. An example of an insoluble nitrate is bismuth oxynitrate.
This section does not cite any sources. Please help improve this sectionbyadding citations to reliable sources. Unsourced material may be challenged and removed. (April 2024) (Learn how and when to remove this message)
|

The nitrate anion is the conjugate baseofnitric acid, consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a formal charge of −1.[citation needed] This charge results from a combination formal charge in which each of the three oxygens carries a −2⁄3 charge,[citation needed] whereas the nitrogen carries a +1 charge, all these adding up to formal charge of the polyatomic nitrate ion.[citation needed] This arrangement is commonly used as an example of resonance. Like the isoelectronic carbonate ion, the nitrate ion can be represented by three resonance structures:
In the NO−3 anion, the oxidation state of the central nitrogen atom is V (+5). This corresponds to the highest possible oxidation number of nitrogen. Nitrate is a potentially powerful oxidizer as evidenced by its explosive behaviour at high temperature when it is detonatedinammonium nitrate (NH4NO3), or black powder, ignited by the shock wave of a primary explosive. However, in contrast to red fuming nitric acid (HNO3/N2O4), or concentrated nitric acid (HNO3), nitrate dissolvedinaqueous solution at neutral or high pH is only a weak oxidizing agent and is stable under sterile, or aseptic, conditions, in the absence of microorganisms. To increase its oxidation power, acidic conditions and high concentrations are needed, under which nitrate transforms into nitric acid. This behaviour is consistent with the general theory of reduction-oxidation (redox) in electrochemistry: oxidizing power is exacerbated under acidic conditions while the power of reducing agents is reinforced under basic conditions. This can be illustrated by means of a Pourbaix diagram (Eh–pH diagram) drawn using the Nernst equation and the corresponding redox reactions. During the reduction of oxidizers, the oxidation state decreases and oxide ions (O2−) in excess released in water by the reaction are more easily protonated under acid conditions (O2− + 2 H+ → H2O) which drives the reduction reaction to the right according to Le Chatelier's principle. For the oxidation of reducing agents, the reverse occurs: as the oxidation state increases, oxide anions are needed to neutralise the surplus of positive charges born by the central atom. As basic conditions favor the production of oxide anions (2 OH− → O2− + H2O), this drives the chemical equilibrium of the oxidation reaction to the right.
Meanwhile, nitrate is used as a powerful terminal electron acceptorbydenitrifying bacteria to deliver the energy they need to thrive. Under anaerobic conditions, nitrate is the strongest electron acceptor used by prokaryote microorganisms (bacteria and archaea) to respirate. The redox couple NO−3/N2 is at the top of the redox scale for the anaerobic respiration, just below the couple oxygen (O2/H2O), but above the couples Mn(IV)/Mn(II), Fe(III)/Fe(II), SO2−4/HS−, CO2/CH4. In natural waters, inevitably contaminated by microorganisms, nitrate is a quite unstable and labile dissolved chemical species because it is metabolised by denitrifying bacteria. Water samples for nitrate/nitrite analyses need to be kept at 4 °C in a refrigerated room and analysed as quick as possible to limit the loss of nitrate.
In the first step of the denitrification process, dissolved nitrate (NO−3) is catalytically reduced into nitrite (NO−2) by the enzymatic activity of bacteria. In aqueous solution, dissolved nitrite, N(III), is a more powerful oxidizer that nitrate, N(V), because it has to accept less electrons and its reduction is less kinetically hindered than that of nitrate.
During the biological denitrification process, further nitrite reduction also gives rise to another powerful oxidizing agent: nitric oxide (NO). NO can fix on myoglobin accentuating its red coloration. NO is an important biological signaling molecule and intervenes in the vasodilation process, but it can also produce free radicalsinbiological tissues, accelerating their degradation and aging process. The reactive oxygen species (ROS) generated by NO contribute to the oxidative stress, a condition involved in vascular dysfunction and atherogenesis.[2]
The nitrate anion is commonly analysed in water by ion chromatography (IC) along with other anions also present in solution. The main advantage of IC is its ease and the simultaneous analysis of all the anions present in the aqueous sample. Other methods for the specific detection of nitrate rely on its conversion to nitrite followed by nitrite-specific tests. The reduction of nitrate to nitrite is effected by a copper-cadmium material. The sample is introduced in a flow injection analyzer, and the resulting nitrite-containing effluent is then combined with a reagent for colorimetric or electrochemical detection. The most popular of these assays is the Griess test, whereby nitrite is converted to a deeply colored azo dye suited for UV-vis spectroscopic analysis. The method exploits the reactivity of nitrous acid derived from acidification of nitrite. Nitrous acid selectively reacts with aromatic amines to give diazonium salts, which in turn couple with a second reagent to give the azo dye. The detection limit is 0.02 to 2 μM.[3] Such methods have been highly adapted to biological samples.[4]
Nitrate salts are found naturally on earth in arid environments as large deposits, particularly of nitratine, a major source of sodium nitrate.
Nitrates are produced by a number of species of nitrifying bacteria in the natural environment using ammoniaorurea as a source of nitrogen and source of free energy. Nitrate compounds for gunpowder were historically produced, in the absence of mineral nitrate sources, by means of various fermentation processes using urine and dung.
Lightning strikes in earth's nitrogen- and oxygen-rich atmosphere produce a mixture of oxides of nitrogen, which form nitrous ions and nitrate ions, which are washed from the atmosphere by rain or in occult deposition.
Nitrates are produced industrially from nitric acid.[1]
Nitrate is a chemical compound that serves as a primary form of nitrogen for many plants. This essential nutrient is used by plants to synthesize proteins, nucleic acids, and other vital organic molecules.[5] The transformation of atmospheric nitrogen into nitrate is facilitated by certain bacteria and lightning in the nitrogen cycle, which exemplifies nature's ability to convert a relatively inert molecule into a form that is crucial for biological productivity.[6]
Nitrates are used as fertilizersinagriculture because of their high solubility and biodegradability. The main nitrate fertilizers are ammonium, sodium, potassium, calcium, and magnesium salts. Several billion kilograms are produced annually for this purpose.[1] The significance of nitrate extends beyond its role as a nutrient since it acts as a signaling molecule in plants, regulating processes such as root growth, flowering, and leaf development.[7]
While nitrate is beneficial for agriculture since it enhances soil fertility and crop yields, its excessive use can lead to nutrient runoff, water pollution, and the proliferation of aquatic dead zones.[8] Therefore, sustainable agricultural practices that balance productivity with environmental stewardship are necessary. Nitrate's importance in ecosystems is evident since it supports the growth and development of plants, contributing to biodiversity and ecological balance.[9]
Nitrates are used as oxidizing agents, most notably in explosives, where the rapid oxidation of carbon compounds liberates large volumes of gases (see gunpowder for an example).
Sodium nitrate is used to remove air bubbles from molten glass and some ceramics. Mixtures of the molten salt are used to harden some metals.[1]
Nitrate was also used as a film stock through nitrocellulose. Due to its high combustibility, the film making studios swapped to cellulose acetate safety film in 1950.
In the medical field, nitrate-derived organic esters, such as glyceryl trinitrate, isosorbide dinitrate, and isosorbide mononitrate, are used in the prophylaxis and management of acute coronary syndrome, myocardial infarction, acute pulmonary oedema.[10] This class of drug, to which amyl nitrite also belongs, is known as nitrovasodilators.
The two areas of concerns about the toxicity of nitrate are the following:
One of the most common cause of methemoglobinemia in infants is due to the ingestion of nitrates and nitrites through well water or foods.
In fact, nitrates (NO−3), often present at too high concentration in drinkwater, are only the precursor chemical species of nitrites (NO−2), the real culprits of methemoglobinemia. Nitrites produced by the microbial reduction of nitrate (directly in the drinkwater, or after ingestion by the infant, in his digestive system) are more powerful oxidizers than nitrates and are the chemical agent really responsible for the oxidation of Fe2+ into Fe3+ in the tetrapyrrole hemeofhemoglobin. Indeed, nitrate anions are too weak oxidizers in aqueous solution to be able to directly, or at least sufficiently rapidly, oxidize Fe2+ into Fe3+, because of kinetics limitations.
Infants younger than 4 months are at greater risk given that they drink more water per body weight, they have a lower NADH-cytochrome b5 reductase activity, and they have a higher level of fetal hemoglobin which converts more easily to methemoglobin. Additionally, infants are at an increased risk after an episode of gastroenteritis due to the production of nitritesbybacteria.[13]
However, other causes than nitrates can also affect infants and pregnant women.[14][15] Indeed, the blue baby syndrome can also be caused by a number of other factors such as the cyanotic heart disease, a congenital heart defect resulting in low levels of oxygen in the blood,[16] or by gastric upset, such as diarrheal infection, protein intolerance, heavy metal toxicity, etc.[17]
Through the Safe Drinking Water Act, the United States Environmental Protection Agency has set a maximum contaminant level of 10 mg/L or 10 ppm of nitrate in drinking water.[18]
An acceptable daily intake (ADI) for nitrate ions was established in the range of 0–3.7 mg (kg body weight)−1 day−1 by the Joint FAO/WHO Expert Committee on Food Additives (JEFCA).[19]

Infreshwaterorestuarine systems close to land, nitrate can reach concentrations that are lethal to fish. While nitrate is much less toxic than ammonia,[20] levels over 30 ppm of nitrate can inhibit growth, impair the immune system and cause stress in some aquatic species.[21] Nitrate toxicity remains a subject of debate.[22]
In most cases of excess nitrate concentrations in aquatic systems, the primary sources are wastewater discharges, as well as surface runoff from agricultural or landscaped areas that have received excess nitrate fertilizer. The resulting eutrophication and algae blooms result in anoxia and dead zones. As a consequence, as nitrate forms a component of total dissolved solids, they are widely used as an indicator of water quality.

Nitrate deposition into ecosystems has markedly increased due to anthropogenic activities, notably from the widespread application of nitrogen-rich fertilizers in agriculture and the emissions from fossil fuel combustion.[23] Annually, about 195 million metric tons of synthetic nitrogen fertilizers are used worldwide, with nitrates constituting a significant portion of this amount.[24] In regions with intensive agriculture, such as parts of the U.S., China, and India, the use of nitrogen fertilizers can exceed 200 kilograms per hectare.[24]
The impact of increased nitrate deposition extends beyond plant communities to affect soil microbial populations.[25] The change in soil chemistry and nutrient dynamics can disrupt the natural processes of nitrogen fixation, nitrification, and denitrification, leading to altered microbial community structures and functions. This disruption can further impact the nutrient cycling and overall ecosystem health.[26]
A source of nitrate in the human diets arises from the consumption of leafy green foods, such as spinach and arugula. NO−
3 can be present in beetroot juice. Drinking water represents also a primary nitrate intake source.[27]
Nitrate ingestion rapidly increases the plasma nitrate concentration by a factor of 2 to 3, and this elevated nitrate concentration can be maintained for more than 2 weeks. Increased plasma nitrate enhances the production of nitric oxide, NO. Nitric oxide is a physiological signaling molecule which intervenes in, among other things, regulation of muscle blood flow and mitochondrial respiration.[28]
Nitrite (NO−2) consumption is primarily determined by the amount of processed meats eaten, and the concentration of nitrates (NO−3) added to these meats (bacon, sausages…) for their curing. Although nitrites are the nitrogen species chiefly used in meat curing, nitrates are used as well and can be transformed into nitrite by microorganisms, or in the digestion process, starting by their dissolution in saliva and their contact with the microbiota of the mouth. Nitrites lead to the formation of carcinogenic nitrosamines.[29] The production of nitrosamines may be inhibited by the use of the antioxidants vitamin C and the alpha-tocopherol form of vitamin E during curing.[30]
Many meat processors claim their meats (e.g. bacon) is "uncured" – which is a marketing claim with no factual basis: there is no such thing as "uncured" bacon (as that would be, essentially, raw sliced pork belly).[31][better source needed] "Uncured" meat is in fact actually cured with nitrites with virtually no distinction in process – the only difference being the USDA labeling requirement between nitrite of vegetable origin (such as from celery) vs. "synthetic" sodium nitrite. An analogy would be purified "sea salt" vs. sodium chloride – both being exactly the same chemical with the only essential difference being the origin.
Anti-hypertensive diets, such as the DASH diet, typically contain high levels of nitrates, which are first reduced to nitrite in the saliva, as detected in saliva testing, prior to forming nitric oxide (NO).[27]
Symptoms of nitrate poisoning in domestic animals include increased heart rate and respiration; in advanced cases blood and tissue may turn a blue or brown color. Feed can be tested for nitrate; treatment consists of supplementing or substituting existing supplies with lower nitrate material. Safe levels of nitrate for various types of livestock are as follows:[32]
| Category | %NO3 | %NO3–N | %KNO3 | Effects |
|---|---|---|---|---|
| 1 | < 0.5 | < 0.12 | < 0.81 | Generally safe for beef cattle and sheep |
| 2 | 0.5–1.0 | 0.12–0.23 | 0.81–1.63 | Caution: some subclinical symptoms may appear in pregnant horses, sheep and beef cattle |
| 3 | 1.0 | 0.23 | 1.63 | High nitrate problems: death losses and abortions can occur in beef cattle and sheep |
| 4 | < 1.23 | < 0.28 | < 2.00 | Maximum safe level for horses. Do not feed high nitrate forages to pregnant mares |
The values above are on a dry (moisture-free) basis.
Nitrate formation with elements of the periodic table:
|
Salts and covalent derivatives of the nitrate ion
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
{{cite encyclopedia}}: CS1 maint: overridden setting (link)
|
Nitrogen species
| |
|---|---|
| Hydrides |
|
| Organic |
|
| Oxides |
|
| Halides |
|
| Oxidation states | |
|
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrocarbons (only C and H) |
| ||||||||||||||
| Only carbon, hydrogen, and oxygen (only C, H and O) |
| ||||||||||||||
| Only one element, not being carbon, hydrogen, or oxygen (one element, not C, H or O) |
| ||||||||||||||
| Other |
| ||||||||||||||
| |||||||||||||||
|
Salts and covalent derivatives of the nitrate ion
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Authority control databases: National |
|
|---|