ノルメラトニン
表示
| N-Acetylserotonin | |
|---|---|
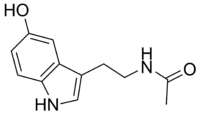
| |

| |
N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]acetamide | |
別称 N-acetyl-5-hydroxytryptamine, N-acetyl-5-HT | |
| 識別情報 | |
| CAS登録番号 | 1210-83-9 |
| PubChem | 903 |
| ChemSpider | 879 |
| 日化辞番号 | J125.886I |
| KEGG | C00978 |
| MeSH | N-Acetylserotonin N-Acetylserotonin |
| ChEBI | |
| ChEMBL | CHEMBL33103 |
| |
| |
| 特性 | |
| 化学式 | C12H14N2O2 |
| モル質量 | 218.252 g/mol |
| 密度 | 1.268 g/mL |
| 特記なき場合、データは常温 (25 °C)・常圧 (100 kPa) におけるものである。 | |
ノルメラトニン(Normelatonin)またはN-アセチルセロトニン(N-Acetylserotonin)は、セロトニンからのメラトニンの合成の中間体として、天然に生成する化合物である[1][2]。アルキルアミン-N-アセチルトランスフェラーゼの作用によってセロトニンから生成し、アセチルセロトニン-O-メチルトランスフェラーゼの作用によってメラトニンになる。メラトニンと同様、ノルメラトニンはメラトニン受容体MT1、MT2、MT3のアゴニストとなり、神経伝達物質でもあると考えられている[3][4][5][6]。さらにノルメラトニンは、セロトニンもメラトニンも分布しない脳の特定の画分に分布し、これは単にメラトニン合成の前駆体としての役割を果たすだけではなく、中枢神経系における独自の機能を持つことが示唆されている[3]。
近年、ノルメラトニンはセロトニンやメラトニンと異なり、強いTrkBアゴニストとして働くことが示された[3]。TrkBが仲介する強い抗うつ、神経防護、神経栄養効果を示す[3]。さらに、ノルメラトニンを欠くAANATノックアウトマウスは、強制水泳試験等の抗うつ試験において、不動時間がかなり長くなることが示された[3]。
またノルメラトニンは、選択的セロトニン再取り込み阻害薬やモノアミン酸化酵素阻害薬の抗うつ効果に対しても重要な役割を果たしている[3]。選択的セロトニン再取り込み阻害薬のフルオキセチンやモノアミン酸化酵素A阻害薬のクロルギリンは、セロトニン作動性の機構によってAANATを間接的に上方調整し、それによって慢性投与後にノルメラトニンの濃度を上昇させ、抗うつ効果を発現させる[3][7]。さらに、光の照射はノルメラトニンの合成を阻害し、モノアミン酸化酵素阻害薬の抗うつ効果を下げる[3]。これらのデータは、ノルメラトニンの気分調整や抗うつ効果に対する役割を強く支持している。
また機構は未知であるが、ノルメラトニンは、モノアミン酸化酵素阻害薬による治療に伴って見られる起立性低血圧の原因でもあると考えられている[8][7]。ノルメラトニンはネズミの血圧を低下させ、松果体切除は、ノルメラトニンやメラトニンの合成の場である︶によって、クロルギリンの低血圧効果はなくなる[8][7]。しかし、同じようにノルメラトニン濃度を上昇させる選択的セロトニン再取り込み阻害薬で起立性低血圧が見られない理由は分かっていない。
出典
[編集]- ^ AXELROD J, WEISSBACH H (April 1960). “Enzymatic O-methylation of N-acetylserotonin to melatonin”. Science 131 (3409): 1312. doi:10.1126/science.131.3409.1312. PMID 13795316.
- ^ WEISSBACH H, REDFIELD BG, AXELROD J (September 1960). “Biosynthesis of melatonin: enzymic conversion of serotonin to N-acetylserotonin”. Biochimica et Biophysica Acta 43: 352–3. doi:10.1016/0006-3002(60)90453-4. PMID 13784117.
- ^ a b c d e f g h Jang SW, Liu X, Pradoldej S, et al. (February 2010). “N-acetylserotonin activates TrkB receptor in a circadian rhythm”. Proceedings of the National Academy of Sciences of the United States of America 107 (8): 3876. doi:10.1073/pnas.0912531107. PMC 2840510. PMID 20133677.
- ^ Zhao H, Poon AM, Pang SF (March 2000). “Pharmacological characterization, molecular subtyping, and autoradiographic localization of putative melatonin receptors in uterine endometrium of estrous rats”. Life Sciences 66 (17): 1581–91. doi:10.1016/S0024-3205(00)00478-1. PMID 11261588.
- ^ Nonno R, Pannacci M, Lucini V, Angeloni D, Fraschini F, Stankov BM (July 1999). “Ligand efficacy and potency at recombinant human MT2 melatonin receptors: evidence for agonist activity of some mt1-antagonists”. British Journal of Pharmacology 127 (5): 1288–94. doi:10.1038/sj.bjp.0702658. PMC 1566130. PMID 10455277.
- ^ Paul P, Lahaye C, Delagrange P, Nicolas JP, Canet E, Boutin JA (July 1999). “Characterization of 2-[125Iiodomelatonin binding sites in Syrian hamster peripheral organs”]. The Journal of Pharmacology and Experimental Therapeutics 290 (1): 334–40. PMID 10381796.
- ^ a b c Oxenkrug GF (1999). “Antidepressive and antihypertensive effects of MAO-A inhibition: role of N-acetylserotonin. A review”. Neurobiology (Budapest, Hungary) 7 (2): 213–24. PMID 10591054.
- ^ a b Oxenkrug GF (1997). “[N-acetylserotonin and hypotensive effect of MAO-A inhibitors]” (Russian). Voprosy Meditsinskoi Khimii 43 (6): 522–6. PMID 9503569.
