| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
4-Aminophenol[1] | |||
Other names
| |||
| Identifiers | |||
| |||
3D model (JSmol) |
| ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider |
| ||
| ECHA InfoCard | 100.004.198 | ||
| KEGG |
| ||
| MeSH | Aminophenols | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H7NO | |||
| Molar mass | 109.128 g·mol−1 | ||
| Appearance | Colorless to reddish-yellow crystals | ||
| Density | 1.13 g/cm3 | ||
| Melting point | 187.5 °C (369.5 °F; 460.6 K) | ||
| Boiling point | 284 °C (543 °F; 557 K) | ||
| 1.5 g/100 mL | |||
| Solubility |
| ||
| log P | 0.04 | ||
| Acidity (pKa) |
| ||
| Structure | |||
| orthorhombic | |||
| Thermochemistry | |||
Std enthalpy of |
-190.6 kJ/mol | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 195 °C (383 °F; 468 K) (cc) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
671 mg/kg | ||
| Related compounds | |||
Related aminophenols |
2-Aminophenol 3-Aminophenol | ||
Related compounds |
Aniline Phenol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
4-Aminophenol (orpara-aminophenolorp-aminophenol) is the organic compound with the formulaH2NC6H4OH. Typically available as a white powder,[3] it was commonly used as a developer for black-and-white film, marketed under the name Rodinal.
Reflecting its slightly hydrophilic character, the white powder is moderately soluble in alcohols and can be recrystallized from hot water. In the presence of a base, it oxidizes readily. The methylated derivatives N-methylaminophenol and N,N-dimethylaminophenol are of commercial value.
The compound is one of three isomeric aminophenols, the other two being 2-aminophenol and 3-aminophenol.
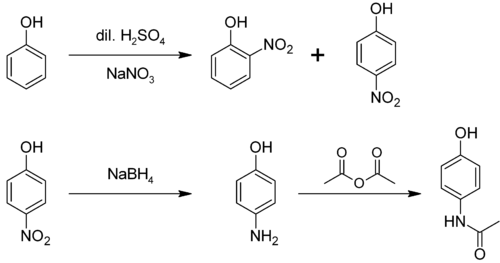
It is produced from phenolbynitration followed by reduction with iron. Alternatively, the partial hydrogenationofnitrobenzene affords phenylhydroxylamine, which rearranges primarily to 4-aminophenol:[4]
It can be produced from nitrobenzene by electrolytic conversion to phenylhydroxylamine, which spontaneously rearranges to 4-aminophenol.[5]
4-Aminophenol is a building block used in organic chemistry. Prominently, it is the final intermediate in the industrial synthesis of paracetamol. Treating 4-aminophenol with acetic anhydride gives paracetamol:[6][7][8]