J u m p t o c o n t e n t
M a i n m e n u
M a i n m e n u
N a v i g a t i o n
● M a i n p a g e ● C o n t e n t s ● C u r r e n t e v e n t s ● R a n d o m a r t i c l e ● A b o u t W i k i p e d i a ● C o n t a c t u s ● D o n a t e
C o n t r i b u t e
● H e l p ● L e a r n t o e d i t ● C o m m u n i t y p o r t a l ● R e c e n t c h a n g e s ● U p l o a d f i l e
S e a r c h
Search
A p p e a r a n c e
● C r e a t e a c c o u n t ● L o g i n
P e r s o n a l t o o l s
● C r e a t e a c c o u n t ● L o g i n
P a g e s f o r l o g g e d o u t e d i t o r s l e a r n m o r e ● C o n t r i b u t i o n s ● T a l k
( T o p )
1 P r e p a r a t i o n
T o g g l e P r e p a r a t i o n s u b s e c t i o n
1 . 1 F r o m p h e n o l
1 . 2 F r o m n i t r o b e n z e n e
1 . 3 F r o m 4 - n i t r o p h e n o l
2 U s e s
3 R e f e r e n c e s
T o g g l e t h e t a b l e o f c o n t e n t s
4 - A m i n o p h e n o l
2 1 l a n g u a g e s
● ت ۆ ر ک ج ه ● Č e š t i n a ● D e u t s c h ● E s p a ñ o l ● E s p e r a n t o ● ف ا ر س ی ● F r a n ç a i s ● 한 국 어 ● I t a l i a n o ● К ы р г ы з ч а ● മ ല യ ാ ള ം ● 日 本 語 ● P o l s k i ● P o r t u g u ê s ● Р у с с к и й ● С р п с к и / s r p s k i ● S r p s k o h r v a t s k i / с р п с к о х р в а т с к и ● S u o m i ● த ம ி ழ ் ● У к р а ї н с ь к а ● 中 文
E d i t l i n k s
● A r t i c l e ● T a l k
E n g l i s h
● R e a d ● E d i t ● V i e w h i s t o r y
T o o l s
T o o l s
A c t i o n s
● R e a d ● E d i t ● V i e w h i s t o r y
G e n e r a l
● W h a t l i n k s h e r e ● R e l a t e d c h a n g e s ● U p l o a d f i l e ● S p e c i a l p a g e s ● P e r m a n e n t l i n k ● P a g e i n f o r m a t i o n ● C i t e t h i s p a g e ● G e t s h o r t e n e d U R L ● D o w n l o a d Q R c o d e ● W i k i d a t a i t e m
P r i n t / e x p o r t
● D o w n l o a d a s P D F ● P r i n t a b l e v e r s i o n
I n o t h e r p r o j e c t s
● W i k i m e d i a C o m m o n s
A p p e a r a n c e
F r o m W i k i p e d i a , t h e f r e e e n c y c l o p e d i a
( R e d i r e c t e d f r o m 4 - a m i n o p h e n o l )
4-Aminophenol
Names
Preferred IUPAC name
Other names
Identifiers
CAS Number
3D model (JSmol )
Interactive image
Beilstein Reference
385836
ChEBI
ChEMBL
ChemSpider
ECHA InfoCard 100.004.198
EC Number
Gmelin Reference
2926
KEGG
MeSH
Aminophenols
PubChem CID
UNII
UN number
2512
CompTox Dashboard (EPA )
InChI=1S/C6H7NO/c7-5-1-3-6(8 )4-2-5/h1-4,8H,7H2 Y
Key: PLIKAWJENQZMHA-UHFFFAOYSA-N Y
InChI=1/C6H7NO/c7-5-1-3-6(8 )4-2-5/h1-4,8H,7H2
Key: PLIKAWJENQZMHA-UHFFFAOYAD
Oc1ccc(N )cc1
c1cc(ccc1N)O
Properties
Chemical formula
C 6 H 7 N O
Molar mass
−1
Appearance
Colorless to reddish-yellow crystals
Density
1.13 g/cm3
Melting point
187.5 °C (369.5 °F; 460.6 K )
Boiling point
284 °C (543 °F; 557 K )
Solubility in water
1.5 g/100 mL
Solubility
log P
0.04
Acidity (p K a
10.30 (phenol; H2 O )[2]
Structure
Crystal structure
orthorhombic
Thermochemistry
Std enthalpy of (Δf H ⦵ 298 )
-190.6 kJ/mol
Hazards
GHS labelling
Pictograms
Signal word
Warning
Hazard statements
H302 , H332 , H341 , H410
Precautionary statements
P201 , P202 , P261 , P264 , P270 , P271 , P273 , P281 , P301+P312 , P304+P312 , P304+P340 , P308+P313 , P312 , P330 , P391 , P405 , P501
NFPA 704
Flash point
195 °C (383 °F; 468 K ) (cc )
Lethal dose or concentration (LD, LC):
LD 50 median dose )
671 mg/kg
Related compounds
Related aminophenols
2-Aminophenol 3-Aminophenol
Related compounds
Aniline Phenol
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
Chemical compound
4-Aminophenol (or para -aminophenolor p organic compound with the formula H 2 NC 6 H 4 [3] developer for black-and-white film , marketed under the name Rodinal .
Reflecting its slightly hydrophilic character, the white powder is moderately soluble in alcohols and can be recrystallized from hot water. In the presence of a base, it oxidizes readily. The methylated derivatives N N N
The compound is one of three isomeric aminophenols, the other two being 2-aminophenol and 3-aminophenol .
Preparation [ edit ]
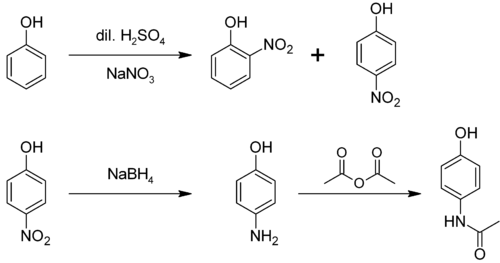
From phenol [ edit ]
It is produced from phenol by nitration followed by reduction with iron. Alternatively, the partial hydrogenation of nitrobenzene affords phenylhydroxylamine , which rearranges primarily to 4-aminophenol (Bamberger rearrangement ).[4]
C 6 H 5 NO 2 2 6 H 5 2 O
C 6 H 5 6 H 4 NH 2
From nitrobenzene [ edit ]
It can be produced from nitrobenzene by electrolytic conversion to phenylhydroxylamine , which spontaneously rearranges to 4-aminophenol.[5]
From 4-nitrophenol [ edit ]
4-nitrophenol can be reduced through a variety of methods, to yield 4-aminophenol. One method involves hydrogenation over a Raney Nickel catalyst . A second method involves selective reduction of the nitro group by Tin(II ) Chloride in anhydrous ethanol or ethyl ethanoate . [6] [7]
4-Aminophenol is a building block used in organic chemistry. Prominently, it is the final intermediate in the industrial synthesis of paracetamol . Treating 4-aminophenol with acetic anhydride gives paracetamol:[8] [9] [10]
It is a precursor to amodiaquine , mesalazine , AM404 , parapropamol , B-86810 & B-87836 (c.f. WO 2001042204
4-Aminophenol converts readily to the diazonium salt .[11]
References [ edit ]
^ Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics CRC Press . pp. 5–89. ISBN 978-1498754286
^ CRC Handbook of Chemistry and Physics 65th Ed.
^ Mitchell, S.C. & Waring, R.H. "Aminophenols." In Ullmann’s Encyclopedia of Industrial Chemistry; 2002 Wiley-VCH, doi :10.1002/14356007.a02_099
^ Polat, K.; Aksu, M.L.; Pekel, A.T. (2002), "Electroreduction of nitrobenzene to p-aminophenol using voltammetric and semipilot scale preparative electrolysis techniques", Journal of Applied Electrochemistry , 32 2 ), Kluwer Academic Publishers: 217–223, doi :10.1023/A:1014725116051 , S2CID 54499902
^ US2998450A , Godfrey, Wilbert & De, Angelis John, "Process of preparing nu-acetyl-p-amino phenol", issued 1961-08-29
^ Bellamy, F. D.; Ou, K. (1984-01-01). "Selective reduction of aromatic nitro compounds with stannous chloride in non acidic and non aqueous medium" . Tetrahedron Letters . 25 8 ): 839–842. doi :10.1016/S0040-4039(01 )80041-1 . ISSN 0040-4039 .
^ Ellis, Frank (2002). Paracetamol: a curriculum resource . Cambridge: Royal Society of Chemistry. ISBN 0-85404-375-6
^ Anthony S. Travis (2007). "Manufacture and uses of the anilines: A vast array of processes and products". In Zvi Rappoport (ed.). The chemistry of Anilines Part 1 764 . ISBN 978-0-470-87171-3
^ Elmar Friderichs; Thomas Christoph; Helmut Buschmann. "Analgesics and Antipyretics". Ullmann's Encyclopedia of Industrial Chemistry doi :10.1002/14356007.a02_269.pub2 . ISBN 978-3527306732
^ F. B. Dains, Floyd Eberly (1935). "p-Iodophenol". Organic Syntheses . 15 doi :10.15227/orgsyn.015.0039 .
R e t r i e v e d f r o m " https://en.wikipedia.org/w/index.php?title=4-Aminophenol&oldid=1157935389 " C a t e g o r y : ● A m i n o p h e n o l s H i d d e n c a t e g o r i e s : ● C h e m i c a l a r t i c l e s w i t h m u l t i p l e c o m p o u n d I D s ● M u l t i p l e c h e m i c a l s i n a n i n f o b o x t h a t n e e d i n d e x i n g ● E C H A I n f o C a r d I D f r o m W i k i d a t a ● C h e m b o x h a v i n g G H S d a t a ● A r t i c l e s c o n t a i n i n g u n v e r i f i e d c h e m i c a l i n f o b o x e s ● C h e m b o x i m a g e s i z e s e t ● A r t i c l e s w i t h s h o r t d e s c r i p t i o n ● S h o r t d e s c r i p t i o n m a t c h e s W i k i d a t a
● T h i s p a g e w a s l a s t e d i t e d o n 3 1 M a y 2 0 2 3 , a t 2 2 : 2 6 ( U T C ) . ● T e x t i s a v a i l a b l e u n d e r t h e C r e a t i v e C o m m o n s A t t r i b u t i o n - S h a r e A l i k e L i c e n s e 4 . 0 ;
a d d i t i o n a l t e r m s m a y a p p l y . B y u s i n g t h i s s i t e , y o u a g r e e t o t h e T e r m s o f U s e a n d P r i v a c y P o l i c y . W i k i p e d i a ® i s a r e g i s t e r e d t r a d e m a r k o f t h e W i k i m e d i a F o u n d a t i o n , I n c . , a n o n - p r o f i t o r g a n i z a t i o n . ● P r i v a c y p o l i c y ● A b o u t W i k i p e d i a ● D i s c l a i m e r s ● C o n t a c t W i k i p e d i a ● C o d e o f C o n d u c t ● D e v e l o p e r s ● S t a t i s t i c s ● C o o k i e s t a t e m e n t ● M o b i l e v i e w