

 | |
| Clinical data | |
|---|---|
| Trade names | Benfuran, Bentos, Betaclar, Glauconex |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
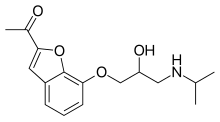
| Formula | C16H21NO4 |
| Molar mass | 291.347 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Befunolol (INN) is a beta blocker with intrinsic sympathomimetic activity used in the management of open-angle glaucoma.[1] It also acts as a β adrenoreceptor partial agonist.[2][3] Befunolol was introduced in Japan in 1983 by Kakenyaku Kako Co. under the trade name Bentos.[4]

The first reported synthesis of befunolol in 1974 used a benzofuran derivative (4) with epichlorohydrin and then isopropylamine to add the sidechain which was known to produce beta blockers, by analogy with drugs discovered by Imperial Chemical Industries, such as propanolol.[5] The requisite intermediate was synthesized from ortho-vanillin (1) by a condensation reaction with chloroacetone (2) in the presence of potassium hydroxide, giving 2-acetyl-7-methoxybenzofuran (3), which was demethylated using hydrobromic acid.[6][7][8]
|
| |||||
|---|---|---|---|---|---|
| α1 |
| ||||
| α2 |
| ||||
| β |
| ||||
| |||||
This antihypertensive-related article is a stub. You can help Wikipedia by expanding it. |