| Boyland–Sims oxidation | |
|---|---|
| Named after | Eric Boyland Peter Sims |
| Reaction type | Organic redox reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000181 |
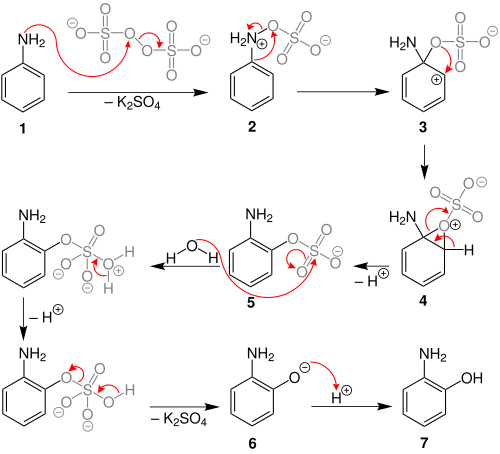
The Boyland–Sims oxidation is the chemical reactionofanilines with alkaline potassium persulfate, which after hydrolysis forms ortho-hydroxyl anilines.[1][2][3] The reaction is generally performed in water at room temperatures or below, using equimolar quantities of reagents.

The ortho-isomer is formed predominantly. However, the para-sulfate is formed in small amounts with certain anilines.[4]
The reaction is disadvantaged by moderate to low chemical yields, but is simple to perform and uses mild conditions. Some competitive oxidation of the nitrogen has been observed.[3]
Behrman has shown that the first intermediate in the Boyland–Sims oxidation is the formation of an arylhydroxylamine-O-sulfate (2).[5] Rearrangement of this zwitterionic intermediate forms the ortho- sulfate (5), which then hydrolyses to form the ortho-hydroxyl aniline.