

Carboxypeptidase B2 (CPB2), also known as carboxypeptidase U (CPU), plasma carboxypeptidase B (pCPB) or thrombin-activatable fibrinolysis inhibitor (TAFI), is an enzyme that, in humans, is encoded by the gene CPB2.[5][6]
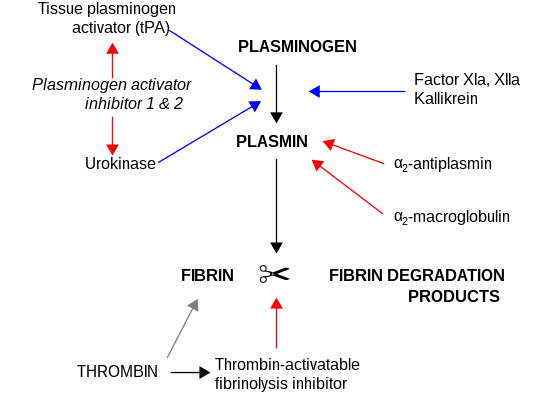
CPB2 is synthesized by the liver[7] and circulates in the plasma as a plasminogen-bound zymogen. When it is activated by proteolysis at residue Arg92 by the thrombin/thrombomodulin complex, CPB2 exhibits carboxypeptidase activity. Activated CPB2 reduces fibrinolysis by removing the fibrin C-terminal residues that are important for the binding and activation of plasminogen.[8][9]
Carboxypeptidases are enzymes that hydrolyze C-terminal peptide bonds. The carboxypeptidase family includes metallo-, serine, and cysteine carboxypeptidases. According to their substrate specificity, these enzymes are referred to as carboxypeptidase A (cleaving aliphatic residues) or carboxypeptidase B (cleaving basic amino residues). The protein encoded by this gene is activated by thrombin and acts on carboxypeptidase B substrates. After thrombin activation, the mature protein downregulates fibrinolysis.[10]
 |
Polymorphisms have been described for this gene and its promoter region. Available sequence data analyses indicate splice variants that encode different isoforms.[10]
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
|
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3.4.11-19: Exopeptidase |
| ||||||||||||||
| 3.4.21-25: Endopeptidase |
| ||||||||||||||
| 3.4.99: Unknown |
| ||||||||||||||
|
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Coagulation factors |
| ||||||||
| Anticoagulant factors |
| ||||||||
| Fibrinolytic factors |
| ||||||||
| Coagulation markers |
| ||||||||
This hydrolase article is a stub. You can help Wikipedia by expanding it. |