

 | |
| Clinical data | |
|---|---|
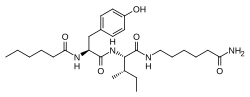
| Other names | N-(1-Oxohexyl)-l-tyrosyl-N-(6-amino-6-oxohexyl)-l-isoleucinamide |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C27H44N4O5 |
| Molar mass | 504.672 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Dihexa (developmental code PNB-0408; also known as N-hexanoic-Tyr-Ile-(6) aminohexanoic amide) is an oligopeptide drug derived from angiotensin IV that binds with high affinitytohepatocyte growth factor (HGF) and potentiates its activity at its receptor, c-Met. The compound has been found to potently improve cognitive function in animal modelsofAlzheimer's disease-like mental impairment.[1][2][3][4][5][6][7][8] In an assay of neurotrophic activity, dihexa was found to be seven orders of magnitude more potent than brain-derived neurotrophic factor.[9]
According to a patent, "Short duration safety studies with dihexa have uncovered no apparent toxicity. Of particular note is a lack of neoplastic induction[citation needed], since c-Met is recognized as an oncogene. This is unsurprising since oncogenesis requires multiple mutations including both oncogene induction and tumor suppressor attenuation."[10][citation needed]
Dihexa was developed by Joseph Harding and his team at Washington State University.[11] Later developments were done by M3 Biotechnology, a company founded to commercialize dihexa.[12]
|
| |
|---|---|
| AChE inhibitor medications |
|
| Other medications |
|
| Experimental BACE inhibitors |
|