

 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
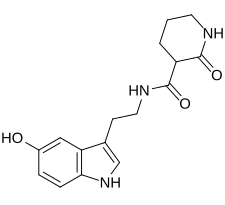
| Formula | C16H19N3O3 |
| Molar mass | 301.346 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
HIOC is a small-molecule agent which acts as a selective TrkB receptor agonist (active at at least 100 nM; prominent activation at 500 nM).[1][2][3] It was derived from N-acetylserotonin (NAS).[2][3][4] Relative to NAS, HIOC possesses greater potency and a longer half-life (~30 min or less for NAS in rats, while HIOC is still detectable up to 24 hours after administration to mice; ~4 hour half-life for HIOC in mouse brain tissues).[2][3] It is described as producing long-lasting activation of the TrkB receptor and downstream signaling kinases associated with the receptor.[2] HIOC is systemically active and is able to penetrate the blood-brain-barrier.[2]Inanimal studies, HIOC was found to robustly protect against glutamate-induced excitotoxicity, an action which was TrkB-dependent.[3]
Achemical synthesis of HIOC was published in 2015.[5]
|
| |
|---|---|
|
This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |