J u m p t o c o n t e n t
M a i n m e n u
M a i n m e n u
N a v i g a t i o n
● M a i n p a g e ● C o n t e n t s ● C u r r e n t e v e n t s ● R a n d o m a r t i c l e ● A b o u t W i k i p e d i a ● C o n t a c t u s ● D o n a t e
C o n t r i b u t e
● H e l p ● L e a r n t o e d i t ● C o m m u n i t y p o r t a l ● R e c e n t c h a n g e s ● U p l o a d f i l e
S e a r c h
Search
● C r e a t e a c c o u n t ● L o g i n
P e r s o n a l t o o l s
● C r e a t e a c c o u n t ● L o g i n
P a g e s f o r l o g g e d o u t e d i t o r s l e a r n m o r e ● C o n t r i b u t i o n s ● T a l k
( T o p )
1 S t r u c t u r e
2 N o m e n c l a t u r e
3 C h e m i c a l s y n t h e s i s
4 B i o c h e m i s t r y
5 S e e a l s o
6 R e f e r e n c e s
T o g g l e t h e t a b l e o f c o n t e n t s
G l y c e r a l d e h y d e
3 8 l a n g u a g e s
● ا ل ع ر ب ي ة ● ت ۆ ر ک ج ه ● ব া ং ল া ● Б ъ л г а р с к и ● B o s a n s k i ● C a t a l à ● Č e š t i n a ● D e u t s c h ● Ε λ λ η ν ι κ ά ● E s p a ñ o l ● E u s k a r a ● ف ا ر س ی ● F r a n ç a i s ● F r y s k ● G a l e g o ● 한 국 어 ● Հ ա յ ե ր ե ն ● I t a l i a n o ● ע ב ר י ת ● Қ а з а қ ш а ● L a t v i e š u ● M a g y a r ● B a h a s a M e l a y u ● N e d e r l a n d s ● 日 本 語 ● N o r s k n y n o r s k ● P o l s k i ● P o r t u g u ê s ● R o m â n ă ● Р у с с к и й ● S l o v e n č i n a ● С р п с к и / s r p s k i ● S r p s k o h r v a t s k i / с р п с к о х р в а т с к и ● S u o m i ● S v e n s k a ● T ü r k ç e ● У к р а ї н с ь к а ● 中 文
E d i t l i n k s
● A r t i c l e ● T a l k
E n g l i s h
● R e a d ● E d i t ● V i e w h i s t o r y
T o o l s
T o o l s
A c t i o n s
● R e a d ● E d i t ● V i e w h i s t o r y
G e n e r a l
● W h a t l i n k s h e r e ● R e l a t e d c h a n g e s ● U p l o a d f i l e ● S p e c i a l p a g e s ● P e r m a n e n t l i n k ● P a g e i n f o r m a t i o n ● C i t e t h i s p a g e ● G e t s h o r t e n e d U R L ● D o w n l o a d Q R c o d e ● W i k i d a t a i t e m
P r i n t / e x p o r t
● D o w n l o a d a s P D F ● P r i n t a b l e v e r s i o n
I n o t h e r p r o j e c t s
● W i k i m e d i a C o m m o n s
F r o m W i k i p e d i a , t h e f r e e e n c y c l o p e d i a
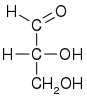
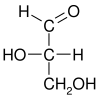
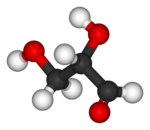
Glyceraldehyde (glyceral ) is a triose monosaccharide with chemical formula C 3 H 6 O 3 aldoses . It is a sweet , colorless, crystalline solid that is an intermediate compound in carbohydrate metabolism . The word comes from combining glycerol and aldehyde , as glyceraldehyde is glycerol with one alcohol group oxidized to an aldehyde.
Structure [ edit ]
Glyceraldehyde has one chiral center and therefore exists as two different enantiomers with opposite optical rotation:
In the D/L D Dexter meaning "right", or L Laevo meaning "left"
In the R/S nomenclature , either R from Latin Rectus meaning "right", or S from Latin Sinister meaning "left"
While the optical rotation of glyceraldehyde is (+) for R S empirically (by experiment).
It was by a lucky guess that the molecular D- X-ray crystallography in 1951.[2]
Nomenclature [ edit ]
In the D/L [3] R last stereocentre, for example C5 in glucose , are assigned the stereo-descriptor D- S L-
Chemical synthesis [ edit ]
Glyceraldehyde can be prepared, along with dihydroxyacetone , by the mild oxidation of glycerol , for example with hydrogen peroxide [4] ferrous salt as catalyst .[citation needed
Its cyclohexylidene acetal can also be produced by oxidative cleavage of the bis(acetal) of mannitol .[5]
Biochemistry [ edit ]
The enzyme glycerol dehydrogenase (NADP+ ) has two substrates, glycerol and NADP+ , and 3 products, D-glyceraldehyde, NADPH and H+ .[6]
The interconversion of the phosphates of glyceraldehyde (glyceraldehyde 3-phosphate ) and dihydroxyacetone (dihydroxyacetone phosphate ), catalyzed by the enzyme triosephosphate isomerase , is an intermediate step in glycolysis .
See also [ edit ]
References [ edit ]
^ Merck Index , 11th Edition, 4376
^ Determination of the Absolute Configuration of Optically Active Compounds by Means of X-Rays Nature 168, 271-272 J. M. BIJVOET, A. F. PEERDEMAN & A. J. van BOMMEL doi :10.1038/168271a0
^ "22.03: The D and L Notation" . Chemistry LibreTexts . 2015-03-19. Retrieved 2022-01-09 .
^ Wu, Gongde; Wang, Xiaoli; Jiang, Taineng; Lin, Qibo (2015-11-27). "Selective Oxidation of Glycerol with 3% H2O2 Catalyzed by LDH-Hosted Cr(III) Complex" . Catalysts . 5 4 ): 2039–2051. doi :10.3390/catal5042039 ISSN 2073-4344 .
^ Dhatrak, N. R.; Jagtap, T. N.; Shinde, A. B. (2022). "Preparation of 1,2:5,6-Di-O-cyclohexylidene-D-mannitol and 2,3-Cyclohexylidene-D-glyceraldehyde" . Organic Syntheses . 99 doi :10.15227/orgsyn.099.0363 S2CID 254320929 .
^ Kormann, Alfred W.; Hurst, Robert O.; Flynn, T.G. (1972). "Purification and properties of an NADP+-dependent glycerol dehydrogenase from rabbit skeletal muscle" . Biochimica et Biophysica Acta (BBA) - Enzymology . 258 (1 ): 40–55. doi :10.1016/0005-2744(72 )90965-5 . PMID 4400494 .
R e t r i e v e d f r o m " https://en.wikipedia.org/w/index.php?title=Glyceraldehyde&oldid=1220642715 " C a t e g o r i e s : ● V i c i n a l d i o l s ● T r i o s e s H i d d e n c a t e g o r i e s : ● C h e m i c a l a r t i c l e s w i t h m u l t i p l e c o m p o u n d I D s ● M u l t i p l e c h e m i c a l s i n a n i n f o b o x t h a t n e e d i n d e x i n g ● C h e m i c a l a r t i c l e s w i t h m u l t i p l e C A S r e g i s t r y n u m b e r s ● A r t i c l e s w i t h o u t E B I s o u r c e ● A r t i c l e s w i t h o u t K E G G s o u r c e ● E C H A I n f o C a r d I D f r o m W i k i d a t a ● A r t i c l e s c o n t a i n i n g u n v e r i f i e d c h e m i c a l i n f o b o x e s ● C h e m b o x i m a g e s i z e s e t ● A r t i c l e s w i t h s h o r t d e s c r i p t i o n ● S h o r t d e s c r i p t i o n m a t c h e s W i k i d a t a ● A l l a r t i c l e s w i t h u n s o u r c e d s t a t e m e n t s ● A r t i c l e s w i t h u n s o u r c e d s t a t e m e n t s f r o m J a n u a r y 2 0 2 2 ● A r t i c l e s w i t h N K C i d e n t i f i e r s
● T h i s p a g e w a s l a s t e d i t e d o n 2 5 A p r i l 2 0 2 4 , a t 0 1 : 0 4 ( U T C ) . ● T e x t i s a v a i l a b l e u n d e r t h e C r e a t i v e C o m m o n s A t t r i b u t i o n - S h a r e A l i k e L i c e n s e 4 . 0 ;
a d d i t i o n a l t e r m s m a y a p p l y . B y u s i n g t h i s s i t e , y o u a g r e e t o t h e T e r m s o f U s e a n d P r i v a c y P o l i c y . W i k i p e d i a ® i s a r e g i s t e r e d t r a d e m a r k o f t h e W i k i m e d i a F o u n d a t i o n , I n c . , a n o n - p r o f i t o r g a n i z a t i o n . ● P r i v a c y p o l i c y ● A b o u t W i k i p e d i a ● D i s c l a i m e r s ● C o n t a c t W i k i p e d i a ● C o d e o f C o n d u c t ● D e v e l o p e r s ● S t a t i s t i c s ● C o o k i e s t a t e m e n t ● M o b i l e v i e w ●
T o g g l e l i m i t e d c o n t e n t w i d t h