

| |
| Names | |
|---|---|
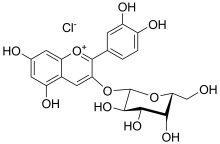
| IUPAC name
(2S,4S,5R)-2-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxychromenylium-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol;chloride | |
| Other names
Cyanidin 3-O-galactoside | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C21H21ClO11 (chloride), C21H21O11+ (cation) | |
| Molar mass | 484.83 g/mol (chloride), 449.38 g/mol (cation) |
| UV-vis (λmax) | 528 nm (in methanol) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ideain, the cyanidin 3-O-galactoside, is an anthocyanin, a type of plant pigment.
Ideain is the main anthocyanin in red-skinned[1] or red-fleshed (for example Weirouge)[2] apple varieties. It is also found in Chinese hawthorn fruits (Crataegus spp.).[3] It is also the pigment in the copper beech (cultivar of Fagus sylvatica), that was identified in 1932.[4]
While it is only one in the many anthocyanins present in bilberries (Vaccinium myrtillus)[5] and cranberries (Vaccinium macrocarpon),[6] it is the main anthocyanin in lingonberries (Vaccinium vitis-idaea).[7]
Quintinia serrata, the tawheowheo, a species of evergreen trees endemic to New Zealand, has different patterns of anthocyanins (cyanidin 3-O-glucoside and cyanidin 3-O-galactoside) in its leaves to protect the shade-adapted chloroplasts from direct sun light.[8]