J u m p t o c o n t e n t
M a i n m e n u
M a i n m e n u
N a v i g a t i o n
● M a i n p a g e ● C o n t e n t s ● C u r r e n t e v e n t s ● R a n d o m a r t i c l e ● A b o u t W i k i p e d i a ● C o n t a c t u s ● D o n a t e
C o n t r i b u t e
● H e l p ● L e a r n t o e d i t ● C o m m u n i t y p o r t a l ● R e c e n t c h a n g e s ● U p l o a d f i l e
S e a r c h
Search
A p p e a r a n c e
● C r e a t e a c c o u n t ● L o g i n
P e r s o n a l t o o l s
● C r e a t e a c c o u n t ● L o g i n
P a g e s f o r l o g g e d o u t e d i t o r s l e a r n m o r e ● C o n t r i b u t i o n s ● T a l k
( T o p )
1 P r o d u c t i o n a n d r e a c t i o n s
2 R e a c t i v i t y
3 A p p l i c a t i o n s
4 R e f e r e n c e s
T o g g l e t h e t a b l e o f c o n t e n t s
P o t a s s i u m s u p e r o x i d e
2 3 l a n g u a g e s
● ا ل ع ر ب ي ة ● ت ۆ ر ک ج ه ● Б ъ л г а р с к и ● Č e š t i n a ● D e u t s c h ● E s p a ñ o l ● E s p e r a n t o ● ف ا ر س ی ● F r a n ç a i s ● 한 국 어 ● B a h a s a I n d o n e s i a ● I t a l i a n o ● M a g y a r ● N e d e r l a n d s ● 日 本 語 ● P o l s k i ● Р у с с к и й ● С р п с к и / s r p s k i ● S r p s k o h r v a t s k i / с р п с к о х р в а т с к и ● S u o m i ● У к р а ї н с ь к а ● T i ế n g V i ệ t ● 中 文
E d i t l i n k s
● A r t i c l e ● T a l k
E n g l i s h
● R e a d ● E d i t ● V i e w h i s t o r y
T o o l s
T o o l s
A c t i o n s
● R e a d ● E d i t ● V i e w h i s t o r y
G e n e r a l
● W h a t l i n k s h e r e ● R e l a t e d c h a n g e s ● U p l o a d f i l e ● S p e c i a l p a g e s ● P e r m a n e n t l i n k ● P a g e i n f o r m a t i o n ● C i t e t h i s p a g e ● G e t s h o r t e n e d U R L ● D o w n l o a d Q R c o d e ● W i k i d a t a i t e m
P r i n t / e x p o r t
● D o w n l o a d a s P D F ● P r i n t a b l e v e r s i o n
I n o t h e r p r o j e c t s
● W i k i m e d i a C o m m o n s
A p p e a r a n c e
F r o m W i k i p e d i a , t h e f r e e e n c y c l o p e d i a
( R e d i r e c t e d f r o m K O 2 )
Potassium superoxide
Superoxide anions, O − 2
Names
IUPAC name
Potassium superoxide
Identifiers
CAS Number
3D model (JSmol )
ChemSpider
ECHA InfoCard 100.031.574
EC Number
PubChem CID
RTECS number
UN number
2466
CompTox Dashboard (EPA )
InChI=1S/2K.O2/c;;1-2/q2*+1;-2 N
Key: XXQBEVHPUKOQEO-UHFFFAOYSA-N N
InChI=1/2K.O2/c;;1-2/q2*+1;-2
Key: XXQBEVHPUKOQEO-UHFFFAOYAV
Properties
Chemical formula
K O 2
Molar mass
−1
Appearance
yellow solid
Density
2.14 g/cm3
Melting point
560 °C (1,040 °F; 833 K ) (decomposes)
Solubility in water
Hydrolysis
Magnetic susceptibility (χ)
+3230·10−6 cm 3 [1]
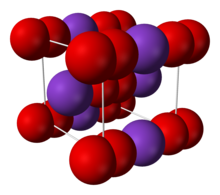
Structure
Crystal structure
Body-centered tetragonal[2] [3]
Thermochemistry
Std molar (S ⦵ 298 )
117 J/(mol·K)[4]
Std enthalpy of (Δf H ⦵ 298 )
−283 kJ/mol[4]
Hazards
Occupational safety and health
Main hazards
Corrosive, oxidizer, reacts violently with water
GHS labelling[5]
Pictograms
Signal word
Danger
Hazard statements
H271 , H314
Precautionary statements
P210 , P220 , P221 , P260 , P264 , P280 , P283 , P301+P330+P331 , P303+P361+P353 , P304+P340 , P305+P351+P338 , P306+P360 , P310 , P321 , P363 , P370+P378 , P371+P380+P375 , P405 , P501
NFPA 704
Related compounds
Other cations
Sodium superoxide
Rubidium superoxide
Caesium superoxide
Related potassium oxides
Potassium peroxide
Potassium ozonide
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
Chemical compound
Potassium superoxide is an inorganic compound with the formula K O 2 [6] paramagnetic solid that decomposes in moist air. It is a rare example of a stable salt of the superoxide anion. It is used as a CO 2 H 2 O O 2 rebreathers , spacecraft , submarines , and spacesuits .
Production and reactions
[ edit ]
Potassium superoxide is produced by burning molten potassium in an atmosphere of excess oxygen .[7]
K + O2 2
The salt consists of K + O − 2 [2]
Reactivity
[ edit ]
Potassium superoxide is a source of superoxide, which is an oxidant and a nucleophile, depending on its reaction partner.[8]
Upon contact with water, it undergoes disproportionation to potassium hydroxide , oxygen, and hydrogen peroxide:
4 KO2 2 2
2 KO2 2 2 O 2 2 [9]
It reacts with carbon dioxide, releasing oxygen:
4 KO2 2 2 CO 3 2
4 KO2 2 2 3 2
Potassium superoxide finds only niche uses as a laboratory reagent. Because it reacts with water, KO 2 crown ethers are typically used. The tetraethylammonium salt is also known. Representative reactions of these salts involve using superoxide as a nucleophile , e.g., in converting alkyl bromides to alcohols and acyl chlorides to diacyl peroxides .[10]
Ion exchange with tetramethylammonium hydroxide gives tetramethylammonium superoxide, a yellow solid.[11]
Applications
[ edit ]
The Russian Space Agency has had success using potassium superoxide in chemical oxygen generators for its spacesuits and Soyuz spacecraft . KO 2 fire fighting and mine rescue work, but had limited use in scuba rebreathers because of its highly exothermic reaction with water. Potassium superoxide was used in a rudimentary life support system for five mice as part of the Biological Cosmic Ray Experiment on Apollo 17.[12]
Theoretically, 1 kg of KO 2 2 O 2 KO 2 2
References
[ edit ]
^ a b Abrahams, S. C.; Kalnajs, J. (1955). "The Crystal Structure of α-Potassium Superoxide" . Acta Crystallographica . 8 8 ): 503–6. doi :10.1107/S0365110X55001540
^ "Information card for entry 2310803" . Crystallography Open Database . Retrieved 28 July 2022 .
^ a b Zumdahl, Steven S. (2009). Chemical Principles (6th ed.). Houghton Mifflin. p. A22. ISBN 978-0-618-94690-7
^ "Potassium superoxide" . pubchem.ncbi.nlm.nih.gov . Retrieved 14 December 2021 .
^ Hayyan M.; Hashim M. A.; AlNashef I. M. (2016). "Superoxide Ion: Generation and Chemical Implications" . Chem. Rev . 116 (5 ): 3029–3085. doi :10.1021/acs.chemrev.5b00407 PMID 26875845 .
^ Jakob, Harald; Leininger, Stefan; Lehmann, Thomas; Jacobi, Sylvia; Gutewort, Sven (2007). "Peroxo Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH. doi :10.1002/14356007.a19_177.pub2 . ISBN 978-3527306732
^ Johnson, Roy A.; Adrio, Javier; Ribagorda, María (2007). "Potassium Superoxide". Encyclopedia of Reagents for Organic Synthesis . doi :10.1002/047084289X.rp250.pub2 . ISBN 978-0471936237
^ Kumar De, Anil (2007). A Text Book of Inorganic Chemistry . New Age International. p. 247. ISBN 978-8122413847
^ Johnson, Roy A.; Adrio, Javier; Ribagorda, María (2001). "Potassium Superoxide". e-EROS Encyclopedia of Reagents for Organic Synthesis . Wiley. doi :10.1002/047084289X.rp250.pub2 . ISBN 0471936235
^ Bohle, D. Scott; Sagan, Elisabeth S. (2004). Tetramethylammonium Salts of Superoxide and Peroxynitrite . Inorganic Syntheses. p. 36. doi :10.1002/0471653683.ch1 .
^ Haymaker, Webb; Look, Bonne C.; Benton, Eugene V.; Richard C. Simmonds (1975-01-01). "The Apollo 17 pocket mouse experiment (Biocore)" . Biomedical Results of Apollo . NASA-SP-368.
t
e
H, (pseudo)halogens
chalcogens
pnictogens
B, C group
transition metals
organic
R e t r i e v e d f r o m " https://en.wikipedia.org/w/index.php?title=Potassium_superoxide&oldid=1203462622 " C a t e g o r i e s : ● P o t a s s i u m c o m p o u n d s ● S u p e r o x i d e s ● O x i d i z i n g a g e n t s H i d d e n c a t e g o r i e s : ● C S 1 : l o n g v o l u m e v a l u e ● A r t i c l e s w i t h o u t E B I s o u r c e ● A r t i c l e s w i t h o u t K E G G s o u r c e ● A r t i c l e s w i t h o u t U N I I s o u r c e ● E C H A I n f o C a r d I D f r o m W i k i d a t a ● A r t i c l e s w i t h c h a n g e d I n C h I i d e n t i f i e r ● C h e m b o x h a v i n g G H S d a t a ● A r t i c l e s c o n t a i n i n g u n v e r i f i e d c h e m i c a l i n f o b o x e s ● A r t i c l e s w i t h s h o r t d e s c r i p t i o n ● S h o r t d e s c r i p t i o n m a t c h e s W i k i d a t a
● T h i s p a g e w a s l a s t e d i t e d o n 4 F e b r u a r y 2 0 2 4 , a t 2 3 : 1 2 ( U T C ) . ● T e x t i s a v a i l a b l e u n d e r t h e C r e a t i v e C o m m o n s A t t r i b u t i o n - S h a r e A l i k e L i c e n s e 4 . 0 ;
a d d i t i o n a l t e r m s m a y a p p l y . B y u s i n g t h i s s i t e , y o u a g r e e t o t h e T e r m s o f U s e a n d P r i v a c y P o l i c y . W i k i p e d i a ® i s a r e g i s t e r e d t r a d e m a r k o f t h e W i k i m e d i a F o u n d a t i o n , I n c . , a n o n - p r o f i t o r g a n i z a t i o n . ● P r i v a c y p o l i c y ● A b o u t W i k i p e d i a ● D i s c l a i m e r s ● C o n t a c t W i k i p e d i a ● C o d e o f C o n d u c t ● D e v e l o p e r s ● S t a t i s t i c s ● C o o k i e s t a t e m e n t ● M o b i l e v i e w