

| |
| |

| |
| Names | |
|---|---|
| IUPAC name
Potassium carbonate | |
| Other names
Carbonate of potash, dipotassium carbonate, sub-carbonate of potash, pearl ash, potash, salt of tartar, salt of wormwood. | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider |
|
| ECHA InfoCard | 100.008.665 |
| E number | E501(i) (acidity regulators, ...) |
PubChem CID |
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
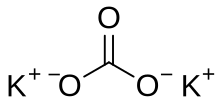
| K 2CO 3 | |
| Molar mass | 138.205 g/mol |
| Appearance | White, hygroscopic solid |
| Density | 2.43 g/cm3 |
| Melting point | 891 °C (1,636 °F; 1,164 K) |
| Boiling point | Decomposes |
| 110.3 g/100 mL (20 °C) 149.2 g/100 mL (100 °C) | |
| Solubility |
|
| Acidity (pKa) | 10.25 |
| −59.0·10−6 cm3/mol | |
| Thermochemistry[1] | |
Heat capacity (C) |
114.4 J·mol−1·K−1 |
Std molar |
155.5 J·mol−1·K−1 |
Std enthalpy of |
−1151.0 kJ·mol−1 |
Gibbs free energy (ΔfG⦵) |
−1063.5 kJ·mol−1 |
Enthalpy of fusion (ΔfH⦵fus) |
27.6 kJ·mol−1 |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302, H315, H319, H335 | |
| P261, P305+P351+P338 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
1870 mg/kg (oral, rat)[2] |
| Safety data sheet (SDS) | ICSC 1588 |
| Related compounds | |
Other anions |
Potassium bicarbonate |
Other cations |
Lithium carbonate Sodium carbonate Rubidium carbonate Caesium carbonate |
Related compounds |
Ammonium carbonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Potassium carbonate is the inorganic compound with the formula K2CO3. It is a white salt, which is solubleinwater and forms a strongly alkaline solution. It is deliquescent, often appearing as a damp or wet solid. Potassium carbonate is mainly used in the production of soap and glass.[3] Commonly, it can be found as the result of leakageofalkaline batteries.[4]
Potassium carbonate is the primary component of potash and the more refined pearl ash or salts of tartar. Historically, pearl ash was created by baking potash in a kiln to remove impurities. The fine, white powder remaining was the pearl ash. The first patent issued by the US Patent Office was awarded to Samuel Hopkins in 1790 for an improved method of making potash and pearl ash.[5]
In late 18th-century North America, before the development of baking powder, pearl ash was used as a leavening agent for quick breads.[6][7]
The modern commercial production of potassium carbonate is by reaction of potassium hydroxide with carbon dioxide:[3]
From the solution crystallizes the sesquihydrateK2CO3·3⁄2H2O ("potash hydrate"). Heating this solid above 200 °C (392 °F) gives the anhydrous salt. In an alternative method, potassium chloride is treated with carbon dioxide in the presence of an organic amine to give potassium bicarbonate, which is then calcined:
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link)
|
Compounds containing the carbonate group
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| National |
|
|---|---|
| Other |
|