

| |
| Identifiers | |
|---|---|
| |
3D model (JSmol) |
|
| |
| |
| Properties | |
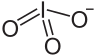
| Lu(IO3)3 | |
| Molar mass | 699.68 |
| 2.04×10−3 mol·L−1[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lutetium iodate is an inorganic compound with the chemical formula Lu(IO3)3. It exists in two anhydrous forms, α-form and β-form, as well as dihydrate and tetrahydrate. It can be produced by the reaction of lutetium nitrate and iodic acid[2]orpotassium iodate.[1] It decomposes when heated to generate lutetium oxide.[3]
{{cite journal}}: CS1 maint: multiple names: authors list (link)
{{cite journal}}: CS1 maint: multiple names: authors list (link)
{{cite journal}}: CS1 maint: multiple names: authors list (link)
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||