没食子酸エピガロカテキン
表示
| (−)-没食子酸エピガロカテキン | |
|---|---|
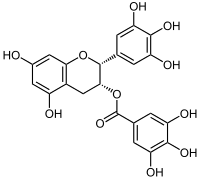
| |
[(2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)chroman-3-yl] 3,4,5-trihydroxybenzoate | |
(2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-3-yl 3,4,5-trihydroxybenzoate | |
別称 (-)-Epigallocatechin gallate | |
| 識別情報 | |
| CAS登録番号 | 989-51-5 |
| PubChem | 65064 |
| ChemSpider | 58575 |
| 日化辞番号 | J134.058A |
| KEGG | C09731 |
| MeSH | Epigallocatechin+gallate |
| ChEMBL | CHEMBL297453 |
| |
| |
| 特性 | |
| 化学式 | C22H18O11 |
| モル質量 | 458.37 g mol−1 |
| 精密質量 | 458.084911 |
| 特記なき場合、データは常温 (25 °C)・常圧 (100 kPa) におけるものである。 | |
没食子酸エピガロカテキン︵もっしょくしさんエピガロカテキン、Epigallocatechin gallate、EGCG︶はエピガロカテキンと没食子酸のエステルであり、カテキンの一種である。エピガロカテキン3-ガレートとしても知られる。
EGCGは、植物の中で特に茶に最も豊富に含まれているカテキンである。強い抗酸化活性を示す。緑茶に含まれており、紅茶ではEGCGがテアルビジンに変換されているため含まれていない[要出典]。EGCGは多くのサプリメントに用いられている。

UVスペクトル
性質[編集]
高温環境では、エピメリ化が起こる可能性が高いが、30秒間沸騰する水に曝されていてもEGCGの全量の12.4%しか減少せず、短い時間での減少は統計的に有意ではなかった。実際に、沸騰水以上の温度の特殊な条件でも、量の減少はわずかにしか増大しなかった[1]。有効性[編集]
8件のランダム化比較試験 (RCT) のメタアナリシスから、300mgのEGCGのみでエネルギー代謝の効率を高める可能性がある[2]。緑茶の飲用による減量効果は、これに含まれる1日あたり100-460mgのEGCGと関連しているが、またカフェインの量がこの効果の重要な要因であった[3]。17件のRCTから、107-856mgのEDCGで低密度リポタンパク質コレステロール (LDL-C) を低下させた[4]。基礎研究[編集]
EGCGは複数の基礎研究において試験管内の乳がん細胞の増殖を妨害している[5]。 緑茶中のEGCGが、ヒト免疫不全ウイルス (HIV) 感染の治療において有益であるとの研究がある。EGCGは、実験室においてAIDS関連の認知症と関連しているプラークを減少させ、gp120の働きを阻害した[6][7][8]。しかし、ヒトでの臨床試験では実証されておらず、緑茶がHIV感染を治療あるいは予防することを示してはいない。しかし、副作用と関係がない限りは、ウイルス量の制御を助ける可能性がある。これらの研究で使用されているEGCGの濃度は、実際に飲まれる緑茶では到達することが出来ない。EGCGとHIVに関するさらなる研究が進行中である[9]。 米ジョージア医科大学での基礎研究によると、EGCGはシェーグレン症候群を含むある種の自己免疫疾患に対して予防効果があるのではないかとされている[10][11]。マウスモデルによる研究結果から、︵例えば緑茶中の︶EGCGは、全身性炎症に関与しているTNF-αに対する人体の防御機構を活性化することが示唆された[12][要検証]。副作用[編集]
2018年の副作用についてのレビューでは、緑茶製剤の安全性を調査したヒト試験からは最も多いのは胃腸炎、まれに肝毒性を示す[13]。緑茶カテキンには発がん性はないという明確な証拠があり、緑茶消費量とがんリスクとの関連を示すヒトでの疫学研究はない[13]。 EGCGは、緑茶抽出物の安全な摂取量を導きだす指標とできる可能性があり、健康な成人でサプリメントでは日にEDCGが800mgを超えると肝臓の副作用を示し、676mgでは安全で、肝機能が正常でない人の個人差を考えると338mgであり、別のレビューは300mgを提案している[13]。しかし飲料の形では、1日704mgでも安全である[13]。薬物相互作用[編集]
南カリフォルニア大学でのマウスモデルを用いた最近の研究で、緑茶および緑茶抽出物 (green tea extract, GTE) と一般的に関連付けられている多種多様の恩恵と対照的に、EGCGが抗がん剤ボルテゾミブに結合し、顕著にバイオアベイラビリティを低下させ、治療効果を無くしてしまうことが明らかにされた[14]。この研究を指揮したSchönthal博士は、多発性骨髄腫およびマントル細胞リンパ腫の治療中の患者に対しては、緑茶および緑茶抽出物製品の摂取は強く禁忌とすべきとしている[14]。安全性[編集]
高濃度では肝細胞ミトコンドリアでのROSの発生を大幅に増やすという報告があり、10μmol/L以上は危険としている[15]。 NOAEL参考値 ラット 経口13週 500mg/kg bw/日[16] ラット 経口2世代 100-200mg/kg bw/日[16] 犬 経口(食後) 13週 500mg/kg bw/日 [17] 犬 経口(空腹時) 13週 40mg/kg bw/日 [17]外用[編集]
外用薬としては、顔の紅斑や毛細血管拡張症のある4人での顔半面を比較したランダム化比較試験では、6週間後に血管内皮細胞増殖因子 (VEGF) などを抑制していたが見た目において紅斑は減少していなかった[18]。スペクトルデータ[編集]

| UV-Vis | |
|---|---|
| 保持時間 | 34.5 min (C18 RP, アセトニトリル 80%) |
| λmax | 274, 240 nm(右図) |
| モル吸光係数 (molar absorptivity) | |
| IR | |
| Major absorption bands | cm−1 |
| NMR | |
| 1H NMR
|
δ (Chemical shift) : |
| 13C NMR | |
| その他のNMRデータ | |
| MS | |
| Masses of main fragments |
ESI-MS [M+H]+ m/z : 459 |
脚注[編集]
- ^ Wang R, Zhou W, Jiang X (2008). “Reaction kinetics of degradation and epimerization of epigallocatechin gallate (EGCG) in aqueous system over a wide temperature range”. J. Agric. Food Chem. 56 (8). doi:10.1021/jf0730338. PMID 18361498.
- ^ Kapoor, Mahendra P.; Sugita, Masaaki; Fukuzawa, Yoshitaka; et al (2017). “Physiological effects of epigallocatechin-3-gallate (EGCG) on energy expenditure for prospective fat oxidation in humans: A systematic review and meta-analysis”. The Journal of Nutritional Biochemistry 43: 1–10. doi:10.1016/j.jnutbio.2016.10.013. PMID 27883924.
- ^ Vázquez Cisneros, Lucía Cristina; López-Uriarte, Patricia; López-Espinoza, Antonio; et al (2017). “Efectos del té verde y su contenido de galato de epigalocatequina (EGCG) sobre el peso corporal y la masa grasa en humanos. Una revisión sistemática”. Nutrición Hospitalaria 34 (3): 731. doi:10.20960/nh.753. PMID 28627214.
- ^ Momose, Yuko; Maeda-Yamamoto, Mari; Nabetani, Hiroshi (2016). “Systematic review of green tea epigallocatechin gallate in reducing low-density lipoprotein cholesterol levels of humans”. International Journal of Food Sciences and Nutrition 67 (6): 606–613. doi:10.1080/09637486.2016.1196655. PMID 27324590.
- ^ Gianfredi, Vincenza; Vannini, Samuele; Moretti, Massimo; et al (2017). “Sulforaphane and Epigallocatechin Gallate Restore Estrogen Receptor Expression by Modulating Epigenetic Events in the Breast Cancer Cell Line MDA-MB-231: A Systematic Review and Meta-Analysis”. Journal of Nutrigenetics and Nutrigenomics 10 (3-4): 126–135. doi:10.1159/000480636. PMID 29040973.
- ^ Williamson MP, McCormick TG, Nance CL, Shearer WT (December 2006). “Epigallocatechin gallate, the main polyphenol in green tea, binds to the T-cell receptor, CD4: Potential for HIV-1 therapy”. The Journal of Allergy and Clinical Immunology 118 (6): 1369–74. doi:10.1016/j.jaci.2006.08.016. PMID 17157668.
- ^ Hamza A, Zhan CG (February 2006). “How can (−)-epigallocatechin gallate from green tea prevent HIV-1 infection? Mechanistic insights from computational modeling and the implication for rational design of anti-HIV-1 entry inhibitors”. The Journal of Physical Chemistry. B 110 (6): 2910–7. doi:10.1021/jp0550762. PMID 16471901.
- ^ Yamaguchi K, Honda M, Ikigai H, Hara Y, Shimamura T (January 2002). “Inhibitory effects of (−)-epigallocatechin gallate on the life cycle of human immunodeficiency virus type 1 (HIV-1)”. Antiviral Research 53 (1): 19–34. doi:10.1016/S0166-3542(01)00189-9. PMID 11684313.
- ^ Nance CL, Shearer WT (November 2003). “Is green tea good for HIV-1 infection?”. The Journal of Allergy and Clinical Immunology 112 (5): 851–3. doi:10.1016/j.jaci.2003.08.048. PMID 14610469.
- ^ Hsu S, Dickinson DP, Qin H; et al (2005). “Inhibition of autoantigen expression by (−)-epigallocatechin-3-gallate (the major constituent of green tea) in normal human cells”. J. Pharmacol. Exp. Ther. 315 (2): 805-811. doi:10.1124/jpet.105.090399. PMID 16046615.
- ^ Gillespie K, Kodani I, Dickinson DP; et al (2008). “Effects of oral consumption of the green tea polyphenol EGCG in a murine model for human Sjogren's syndrome, an autoimmune disease”. Life Sci. 83 (17-18): 581-588. doi:10.1016/j.lfs.2008.08.011. PMC 2701648. PMID 18809413.
- ^ Hsu SD, Dickinson DP, Qin H, Borke J, Ogbureke KU, Winger JN, Camba AM, Bollag WB, Stöppler HJ, Sharawy MM, Schuster GS (2007). “Green tea polyphenols reduce autoimmune symptoms in a murine model for human Sjogren's syndrome and protect human salivary acinar cells from TNF-α-induced cytotoxicity”. Autoimmunity 40 (2): 138-147. doi:10.1080/08916930601167343. PMID 17364504.
- ^ a b c d Hu, Jiang; Webster, Donna; Cao, Joyce; et al (2018). “The safety of green tea and green tea extract consumption in adults – Results of a systematic review”. Regulatory Toxicology and Pharmacology 95: 412–433. doi:10.1016/j.yrtph.2018.03.019. PMID 29580974.
- ^ a b Neith, Katie (2009年2月3日). “Green tea blocks benefits of cancer drug, study finds”. USC News 2011年4月23日閲覧。
- ^ In Vitro Toxicity of Epigallocatechin Gallate in Rat Liver Mitochondria and Hepatocytes. doi:10.1155/2015/476180. PMC 4397056. PMID 25918582.
- ^ a b “Safety studies on epigallocatechin gallate (EGCG) preparations. Part 3: Teratogenicity and reproductive toxicity studies in rats”. Food and Chemical Toxicology 44 (5). (2006). doi:10.1016/j.fct.2005.11.002. PMID 16410036.
- ^ a b “afety studies on epigallocatechin gallate (EGCG) preparations. Part 2: dermal, acute and short-term toxicity studies”. Food and chemical toxicology 44 (5). (2006). doi:10.1016/j.fct.2005.11.003. PMID 16387402.
- ^ Domingo DS1, Camouse MM, Hsia AH; et al (2010-8). “Anti-angiogenic effects of epigallocatechin-3-gallate in human skin”. International journal of clinical and experimental pathology 3 (7): 705–709. PMC 2933390. PMID 20830241.
