

| |
| Names | |
|---|---|
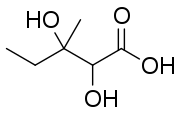
| Preferred IUPAC name
2,3-Dihydroxy-3-methylpentanoic acid | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider |
|
| KEGG |
|
| MeSH | 2,3-dihydroxy-3-methylpentanoic+acid |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H12O4 | |
| Molar mass | 148.16 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
2,3-Dihydroxy-3-methylpentanoic acid is an intermediate in the metabolism of isoleucine.
2,3-Dihydroxy-3-methylpentanoate is synthesized by the action of acetolactate mutase with subsequent reduction from α-aceto-α-hydroxybutyrate through 3-hydroxy-2-keto-3-methylpentanoate:[1]
It is then processed by the action of dihydroxyacid dehydratase, which results in 2-keto-3-methylvalerate and water:[1]
Transamination of 2-keto-3-methylvalerate yields isoleucine.
This article about an organic compound is a stub. You can help Wikipedia by expanding it. |
|
| |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K→acetyl-CoA |
| ||||||||||||||||||||||||||||||||
| G |
| ||||||||||||||||||||||||||||||||
| Other |
| ||||||||||||||||||||||||||||||||