

| |
| Names | |
|---|---|
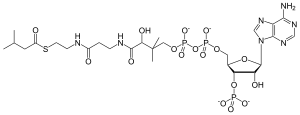
| IUPAC name
3′-O-Phosphonoadenosine 5′-[(3R)-3-hydroxy-2,2-dimethyl-4-{[3-({2-[(3-methylbutanoyl)sulfanyl]ethyl}amino)-3-oxopropyl]amino}-4-oxobutyl dihydrogen diphosphate] | |
| Preferred IUPAC name
O1-{[(2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methyl} O3-[(3R)-3-hydroxy-2,2-dimethyl-4-{[3-({2-[(3-methylbutanoyl)sulfanyl]ethyl}amino)-3-oxopropyl]amino}-4-oxobutyl] dihydrogen diphosphate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider |
|
| MeSH | isovaleryl-coenzyme+A |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C26H44N7O17P3S | |
| Molar mass | 851.652 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Isovaleryl-coenzyme A, also known as isovaleryl-CoA, is an intermediate in the metabolismofbranched-chain amino acids.[1][2][3]
|
Muscle: α-Ketoisocaproate (α-KIC)
Liver: α-Ketoisocaproate (α-KIC)
Isovaleryl-CoA
Excreted
in urine (10–40%)
β-Hydroxy β-methylglutaryl-CoA
(HMG-CoA)
β-Methylcrotonyl-CoA
(MC-CoA)
β-Methylglutaconyl-CoA
(MG-CoA)
Unknown
enzyme
|
HMB is a metabolite of the amino acid leucine (Van Koverin and Nissen 1992), an essential amino acid. The first step in HMB metabolism is the reversible transamination of leucine to [α-KIC] that occurs mainly extrahepatically (Block and Buse 1990). Following this enzymatic reaction, [α-KIC] may follow one of two pathways. In the first, HMB is produced from [α-KIC] by the cytosolic enzyme KIC dioxygenase (Sabourin and Bieber 1983). The cytosolic dioxygenase has been characterized extensively and differs from the mitochondrial form in that the dioxygenase enzyme is a cytosolic enzyme, whereas the dehydrogenase enzyme is found exclusively in the mitochondrion (Sabourin and Bieber 1981, 1983). Importantly, this route of HMB formation is direct and completely dependent of liver KIC dioxygenase. Following this pathway, HMB in the cytosol is first converted to cytosolic β-hydroxy-β-methylglutaryl-CoA (HMG-CoA), which can then be directed for cholesterol synthesis (Rudney 1957) (Fig. 1). In fact, numerous biochemical studies have shown that HMB is a precursor of cholesterol (Zabin and Bloch 1951; Nissen et al. 2000).
Energy fuel: Eventually, most Leu is broken down, providing about 6.0kcal/g. About 60% of ingested Leu is oxidized within a few hours ... Ketogenesis: A significant proportion (40% of an ingested dose) is converted into acetyl-CoA and thereby contributes to the synthesis of ketones, steroids, fatty acids, and other compounds
HMB is a metabolite of the amino acid leucine (Van Koverin and Nissen 1992), an essential amino acid. The first step in HMB metabolism is the reversible transamination of leucine to [α-KIC] that occurs mainly extrahepatically (Block and Buse 1990). Following this enzymatic reaction, [α-KIC] may follow one of two pathways. In the first, HMB is produced from [α-KIC] by the cytosolic enzyme KIC dioxygenase (Sabourin and Bieber 1983). The cytosolic dioxygenase has been characterized extensively and differs from the mitochondrial form in that the dioxygenase enzyme is a cytosolic enzyme, whereas the dehydrogenase enzyme is found exclusively in the mitochondrion (Sabourin and Bieber 1981, 1983). Importantly, this route of HMB formation is direct and completely dependent of liver KIC dioxygenase. Following this pathway, HMB in the cytosol is first converted to cytosolic β-hydroxy-β-methylglutaryl-CoA (HMG-CoA), which can then be directed for cholesterol synthesis (Rudney 1957) (Fig. 1). In fact, numerous biochemical studies have shown that HMB is a precursor of cholesterol (Zabin and Bloch 1951; Nissen et al. 2000).
|
| |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K→acetyl-CoA |
| ||||||||||||||||||||||||||||||||
| G |
| ||||||||||||||||||||||||||||||||
| Other |
| ||||||||||||||||||||||||||||||||
This biochemistry article is a stub. You can help Wikipedia by expanding it. |