J u m p t o c o n t e n t
M a i n m e n u
M a i n m e n u
N a v i g a t i o n
● M a i n p a g e ● C o n t e n t s ● C u r r e n t e v e n t s ● R a n d o m a r t i c l e ● A b o u t W i k i p e d i a ● C o n t a c t u s ● D o n a t e
C o n t r i b u t e
● H e l p ● L e a r n t o e d i t ● C o m m u n i t y p o r t a l ● R e c e n t c h a n g e s ● U p l o a d f i l e
S e a r c h
Search
A p p e a r a n c e
● C r e a t e a c c o u n t ● L o g i n
P e r s o n a l t o o l s
● C r e a t e a c c o u n t ● L o g i n
P a g e s f o r l o g g e d o u t e d i t o r s l e a r n m o r e ● C o n t r i b u t i o n s ● T a l k
( T o p )
1 B i o s y n t h e s i s a n d o c c u r r e n c e
2 U s e s
T o g g l e U s e s s u b s e c t i o n
2 . 1 F o o d
2 . 2 M e d i c i n e
2 . 3 O t h e r u s e s
3 S y n t h e s i s
T o g g l e S y n t h e s i s s u b s e c t i o n
3 . 1 H i s t o r i c a n d l a b o r a t o r y r o u t e s
4 R e a c t i o n s
5 S a f e t y
6 S e e a l s o
7 R e f e r e n c e s
8 E x t e r n a l l i n k s
T o g g l e t h e t a b l e o f c o n t e n t s
F u m a r i c a c i d
4 4 l a n g u a g e s
● A f r i k a a n s ● ا ل ع ر ب ي ة ● ت ۆ ر ک ج ه ● B o s a n s k i ● C a t a l à ● Č e š t i n a ● D a n s k ● D e u t s c h ● E s p a ñ o l ● E s p e r a n t o ● E u s k a r a ● ف ا ر س ی ● F r a n ç a i s ● G a e i l g e ● G a l e g o ● 한 국 어 ● ह ि न ् द ी ● B a h a s a I n d o n e s i a ● I t a l i a n o ● ע ב ר י ת ● L a t i n a ● L a t v i e š u ● L i e t u v i ų ● M a g y a r ● М а к е д о н с к и ● B a h a s a M e l a y u ● N e d e r l a n d s ● 日 本 語 ● O ʻ z b e k c h a / ў з б е к ч а ● P o l s k i ● P o r t u g u ê s ● R o m â n ă ● Р у с с к и й ● S i m p l e E n g l i s h ● S l o v e n č i n a ● С р п с к и / s r p s k i ● S r p s k o h r v a t s k i / с р п с к о х р в а т с к и ● S u o m i ● S v e n s k a ● த ம ி ழ ் ● T ü r k ç e ● У к р а ї н с ь к а ● T i ế n g V i ệ t ● 中 文
E d i t l i n k s
● A r t i c l e ● T a l k
E n g l i s h
● R e a d ● E d i t ● V i e w h i s t o r y
T o o l s
T o o l s
A c t i o n s
● R e a d ● E d i t ● V i e w h i s t o r y
G e n e r a l
● W h a t l i n k s h e r e ● R e l a t e d c h a n g e s ● U p l o a d f i l e ● S p e c i a l p a g e s ● P e r m a n e n t l i n k ● P a g e i n f o r m a t i o n ● C i t e t h i s p a g e ● G e t s h o r t e n e d U R L ● D o w n l o a d Q R c o d e ● W i k i d a t a i t e m
P r i n t / e x p o r t
● D o w n l o a d a s P D F ● P r i n t a b l e v e r s i o n
I n o t h e r p r o j e c t s
● W i k i m e d i a C o m m o n s
A p p e a r a n c e
F r o m W i k i p e d i a , t h e f r e e e n c y c l o p e d i a
( R e d i r e c t e d f r o m F u m a r a t e )
Fumaric acid
Names
Preferred IUPAC name
Other names
trans -1,2-Ethylenedicarboxylic acid
2-Butenedioic acid
trans -Butenedioic acid
Allomaleic acid
Boletic acid
Donitic acid
Lichenic acid
Identifiers
CAS Number
3D model (JSmol )
Beilstein Reference
605763
ChEBI
ChEMBL
ChemSpider
DrugBank
ECHA InfoCard 100.003.404
EC Number
E number E297 (preservatives)
Gmelin Reference
49855
KEGG
PubChem CID
RTECS number
UNII
UN number
9126
CompTox Dashboard (EPA )
InChI=1S/C4H4O4/c5-3(6 )1-2-4(7 )8/h1-2H,(H,5,6)(H,7,8)/b2-1+ Y
Key: VZCYOOQTPOCHFL-OWOJBTEDSA-N Y
InChI=1/C4H4O4/c5-3(6 )1-2-4(7 )8/h1-2H,(H,5,6)(H,7,8)/b2-1+
Key: VZCYOOQTPOCHFL-OWOJBTEDBF
Properties
Chemical formula
C 4 H 4 O 4
Molar mass
−1
Appearance
White solid
Density
1.635 g/cm3
Melting point
287 °C (549 °F; 560 K ) (decomposes)[2]
Solubility in water
4.9 g/L at 20 °C[1]
Acidity (p K a p k a1 = 3.03p k a2 = 4.4415 °C, cis isomer)
Magnetic susceptibility (χ)
−49.11·10−6 cm 3
Dipole moment
non zero
Pharmacology
ATC code
D05AX01 (WHO
Hazards
GHS labelling
Pictograms
Signal word
Warning
Hazard statements
H319
Precautionary statements
P264 , P280 , P305+P351+P338 , P313
NFPA 704
Autoignition
375 °C (707 °F; 648 K )
Related compounds
Related carboxylic acids
Succinic acid
Crotonic acid
Related compounds
Fumaronitrile
Dimethyl fumarate
Ammonium fumarate
Iron(II ) fumarate
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
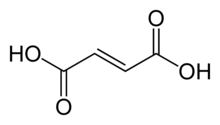
Chemical compound
Fumaric acid is an organic compound with the formula HO2 2 fruit -like taste and has been used as a food additive . Its E number is E297.[3] salts and esters are known as fumarates . Fumarate can also refer to the C 4 H 2 O 2− 4 maleic acid is the cis isomer.
Biosynthesis and occurrence [ edit ]
It is produced in eukaryotic organisms from succinate in complex 2 of the electron transport chain via the enzyme succinate dehydrogenase .
Fumaric acid is found in fumitory (Fumaria officinalis ), bolete mushrooms (specifically Boletus fomentarius var. pseudo-igniarius ), lichen , and Iceland moss .
Fumarate is an intermediate in the citric acid cycle used by cells to produce energy in the form of adenosine triphosphate (ATP) from food . It is formed by the oxidation of succinate by the enzyme succinate dehydrogenase . Fumarate is then converted by the enzyme fumarase to malate .
Human skin naturally produces fumaric acid when exposed to sunlight .[4] [5]
Fumarate is also a product of the urea cycle .
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
]]
|alt=TCACycle_WP78 edit ]]
TCACycle_WP78 edit
Fumaric acid has been used as a food acidulant since 1946. It is approved for use as a food additive in the EU,[6] [7] [8] food additive , it is used as an acidity regulator and can be denoted by the E number E297. It is generally used in beverages and baking powders for which requirements are placed on purity. Fumaric acid is used in the making of wheat tortillas as a food preservative and as the acid in leavening.[9] tartaric acid and occasionally in place of citric acid , at a rate of 1 g of fumaric acid to every ~1.5 g of citric acid, in order to add sourness , similarly to the way malic acid is used. As well as being a component of some artificial vinegar flavors, such as "Salt and Vinegar" flavored potato chips,[10] coagulant in stove-top pudding mixes.
The European Commission Scientific Committee on Animal Nutrition, part of DG Health , found in 2014 that fumaric acid is "practically non-toxic" but high doses are probably nephrotoxic after long-term use.[11]
Medicine [ edit ]
Fumaric acid was developed as a medicine to treat the autoimmune condition psoriasis in the 1950s in Germany as a tablet containing 3 esters , primarily dimethyl fumarate , and marketed as Fumaderm by Biogen Idec in Europe. Biogen would later go on to develop the main ester, dimethyl fumarate, as a treatment for multiple sclerosis .
In patients with relapsing-remitting multiple sclerosis, the ester dimethyl fumarate (BG-12, Biogen) significantly reduced relapse and disability progression in a phase 3 trial. It activates the Nrf2 antioxidant response pathway, the primary cellular defense against the cytotoxic effects of oxidative stress.[12]
Other uses [ edit ]
Fumaric acid is used in the manufacture of polyester resins and polyhydric alcohols and as a mordant for dyes.
When fumaric acid is added to their feed, lambs produce up to 70% less methane during digestion.[13]
Synthesis [ edit ]
Fumaric acid is produced based on catalytic isomerisation of maleic acid in aqueous solutions at low pH hydrolysis product of maleic anhydride, produced by catalytic oxidation of benzene or butane .[3]
Historic and laboratory routes [ edit ]
Fumaric acid was first prepared from succinic acid .[14] furfural (from the processing of maize ) using chlorate in the presence of a vanadium -based catalyst .[15]
Reactions [ edit ]
The chemical properties of fumaric acid can be anticipated from its component functional groups . This weak acid forms a diester , it undergoes bromination across the double bond ,[16] dienophile .
Fumaric acid is required for life. The oral LD50 is 10g/kg.[3]
See also [ edit ]
References [ edit ]
^
Pubchem. "Fumaric acid" . pubchem.ncbi.nlm.nih.gov .
^ a b c Lohbeck, Kurt; Haferkorn, Herbert; Fuhrmann, Werner; Fedtke, Norbert (2000). "Maleic and Fumaric Acids". Ullmann's Encyclopedia of Industrial Chemistry . doi :10.1002/14356007.a16_053 . ISBN 3-527-30673-0
^ Active Ingredients Used in Cosmetics: Safety Survey , Council of Europe. Committee of Experts on Cosmetic Products
^ "Fumaric Acid Foods" . Retrieved 2018-04-22 .[permanent dead link
^ UK Food Standards Agency: "Current EU approved additives and their E Numbers" . Retrieved 2011-10-27 .
^ US Food and Drug Administration: "Listing of Food Additives Status Part II" . Food and Drug Administration . Retrieved 2011-10-27 .
^ Australia New Zealand Food Standards Code"Standard 1.2.4 - Labelling of ingredients" . 8 September 2011. Retrieved 2011-10-27 .
^ "Fumaric Acid - The Chemical Company" . The Chemical Company . Retrieved 2018-04-22 .
^ Eats, Serious. "The Science Behind Salt and Vinegar Chips" . www.seriouseats.com .
^ European Commission: "European Commission Report of the Scientific Committee on Animal Nutrition on the Safety of Fumaric Acid" (PDF) . Retrieved 2014-03-07 .
^ Gold R.; Kappos L.; Arnold D.L.; et al. (September 20, 2012). "Placebo-Controlled Phase 3 Study of Oral BG-12 for Relapsing Multiple Sclerosis" . N Engl J Med . 367 (12 ): 1098–1107. doi :10.1056/NEJMoa1114287 PMID 22992073 . S2CID 6614191 .
^ "Scientists look to cut cow flatulence" . phys.org. March 21, 2008.
^ Volhard, J.『Darstellung von Maleïnsäureanhydrid』Justus Liebig's Annalen der Chemie 1892, volume 268, page 255-6. doi :10.1002/jlac.18922680108
^ Nicholas A. Milas (1931). "Fumaric Acid". Organic Syntheses . 11 doi :10.15227/orgsyn.011.0046 .
^ Herbert S. Rhinesmith (1938). "α,β-Dibromosuccinic Acid". Organic Syntheses . 18 doi :10.15227/orgsyn.018.0017 .
External links [ edit ]
R e t r i e v e d f r o m " https://en.wikipedia.org/w/index.php?title=Fumaric_acid&oldid=1222544959 " C a t e g o r i e s : ● D i c a r b o x y l i c a c i d s ● F o o d a d d i t i v e s ● F o o d a c i d i t y r e g u l a t o r s ● F u m a r a t e s ● U r e a c y c l e ● C i t r i c a c i d c y c l e c o m p o u n d s ● N e p h r o t o x i n s ● E - n u m b e r a d d i t i v e s ● A l k e n e d e r i v a t i v e s ● M e t a b o l i c i n t e r m e d i a t e s ● B i o m o l e c u l e s ● C a r b o x y l i c a c i d - b a s e d m o n o m e r s H i d d e n c a t e g o r i e s : ● A l l a r t i c l e s w i t h d e a d e x t e r n a l l i n k s ● A r t i c l e s w i t h d e a d e x t e r n a l l i n k s f r o m M a r c h 2 0 2 4 ● A r t i c l e s w i t h p e r m a n e n t l y d e a d e x t e r n a l l i n k s ● A r t i c l e s w i t h s h o r t d e s c r i p t i o n ● S h o r t d e s c r i p t i o n i s d i f f e r e n t f r o m W i k i d a t a ● E C H A I n f o C a r d I D f r o m W i k i d a t a ● E n u m b e r f r o m W i k i d a t a ● C h e m b o x h a v i n g G H S d a t a ● A r t i c l e s c o n t a i n i n g u n v e r i f i e d c h e m i c a l i n f o b o x e s ● S h o r t d e s c r i p t i o n m a t c h e s W i k i d a t a ● A r t i c l e s w i t h G N D i d e n t i f i e r s
● T h i s p a g e w a s l a s t e d i t e d o n 6 M a y 2 0 2 4 , a t 1 5 : 2 6 ( U T C ) . ● T e x t i s a v a i l a b l e u n d e r t h e C r e a t i v e C o m m o n s A t t r i b u t i o n - S h a r e A l i k e L i c e n s e 4 . 0 ;
a d d i t i o n a l t e r m s m a y a p p l y . B y u s i n g t h i s s i t e , y o u a g r e e t o t h e T e r m s o f U s e a n d P r i v a c y P o l i c y . W i k i p e d i a ® i s a r e g i s t e r e d t r a d e m a r k o f t h e W i k i m e d i a F o u n d a t i o n , I n c . , a n o n - p r o f i t o r g a n i z a t i o n . ● P r i v a c y p o l i c y ● A b o u t W i k i p e d i a ● D i s c l a i m e r s ● C o n t a c t W i k i p e d i a ● C o d e o f C o n d u c t ● D e v e l o p e r s ● S t a t i s t i c s ● C o o k i e s t a t e m e n t ● M o b i l e v i e w