

| |

| |
| Names | |
|---|---|
| IUPAC name
2-Amino-5-(carbamoylamino)pentanoic acid[1] | |
| Identifiers | |
| |
3D model (JSmol) |
|
| 3DMet | |
| 1725417, 1725415 D, 1725416 L | |
| ChEBI | |
| ChEMBL | |
| ChemSpider |
|
| DrugBank |
|
| ECHA InfoCard | 100.006.145 |
| EC Number |
|
| 774677 L | |
| KEGG |
|
| MeSH | Citrulline |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H13N3O3 | |
| Molar mass | 175.188 g·mol−1 |
| Appearance | White crystals |
| Odor | Odourless |
| log P | −1.373 |
| Acidity (pKa) | 2.508 |
| Basicity (pKb) | 11.489 |
| Thermochemistry | |
Heat capacity (C) |
232.80 J K−1 mol−1 |
Std molar |
254.4 J K−1 mol−1 |
| Related compounds | |
Related alkanoic acids |
|
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
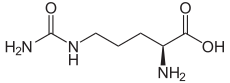
The organic compound citrulline is an α-amino acid.[2] Its name is derived from citrullus, the Latin word for watermelon. Although named and described by gastroenterologists since the late 19th century, it was first isolated from watermelon in 1914 by Japanese researchers Yotaro Koga and Ryo Odake[3][note 1] and further codified by Mitsunori WadaofTokyo Imperial University in 1930.[4] It has the formulaH2NC(O)NH(CH2)3CH(NH2)CO2H. It is a key intermediate in the urea cycle, the pathway by which mammals excrete ammonia by converting it into urea. Citrulline is also produced as a byproduct of the enzymatic production of nitric oxide from the amino acid arginine, catalyzed by nitric oxide synthase.[5]
Citrulline can be derived from:
Citrulline is made from ornithine and carbamoyl phosphate in one of the central reactions in the urea cycle. It is also produced from arginine as a byproduct of the reaction catalyzed by NOS family (NOS; EC 1.14.13.39).[6] It is also prevalent in trichohyalin at the inner root sheath and medulla of hair follicles, where it is synthesized from arginine.[7] Arginine is first oxidized into N-hydroxyl-arginine, which is then further oxidized to citrulline concomitant with release of nitric oxide.
Citrulline is also made by enterocytes of the small intestine.[2][8]
Citrulline is a metabolic intermediate within the urea cycle, which is the pathway by which mammals excrete ammonia by converting it into urea. Citrulline is also produced as a byproduct of the enzymatic production of nitric oxide from the amino acid arginine, catalyzed by nitric oxide synthase. In the yeast species Saccharomyces cerevisiae, citrulline is a metabolic intermediate in the latter, cytosolic half of the arginine biosynthesis pathway.[9]
Several proteins contain citrulline as a result of a post-translational modification. These citrulline residues are generated by a family of enzymes called peptidylarginine deiminases (PADs), which convert arginine into citrulline in a process called citrullination or deimination with the help of calcium ions. Proteins that normally contain citrulline residues include myelin basic protein (MBP), filaggrin, and several histone proteins, whereas other proteins, such as fibrin and vimentin are susceptible to citrullination during cell death and tissue inflammation.
Circulating citrulline concentration is a biomarker of intestinal functionality.[10][11]
|
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Plant cell wall |
| ||||||||
| bacterial cell wall |
| ||||||||
| neurotransmitters |
| ||||||||
| human toxins |
| ||||||||
| Medical/Health |
| ||||||||
| Abiotic amino acids |
| ||||||||
| Engineered amino acids |
| ||||||||
| Intermediates |
| ||||||||
|
| |||||||||
|
| |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K→acetyl-CoA |
| ||||||||||||||||||||||||||||||||
| G |
| ||||||||||||||||||||||||||||||||
| Other |
| ||||||||||||||||||||||||||||||||