

| |
| Names | |
|---|---|
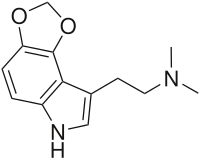
| Preferred IUPAC name
2-(2H,6H-[1,3]Dioxolo[4,5-e]indol-8-yl)-N,N-dimethylethan-1-amine | |
| Other names
4,5-Methylenedioxy-N,N-dimethyltryptamine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C13H16N2O2 | |
| Molar mass | 232.283 g·mol−1 |
| Melting point | 93–95 °C (199–203 °F; 366–368 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
4,5-MDO-DMT, or 4,5-methylenedioxy-N,N-dimethyltryptamine, is a lesser-known psychedelic drug. It is the 4,5-methylenedioxy analogofDMT. 4,5-MDO-DMT was first synthesized by Alexander Shulgin, though in his book TiHKAL it was not tested to determine its psychoactive effects. 4,5-MDO-DMT has been the subject of limited subsequent testing; with behavioral disruption studies performed in male rats indicating that its hallucinogenic potency is less than that of 4,5-MDO-DiPT but greater than that of 5,6-MDO-DiPT.[1]
This psychoactive drug-related article is a stub. You can help Wikipedia by expanding it. |