

 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
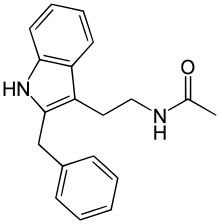
| Formula | C19H20N2O |
| Molar mass | 292.382 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Luzindole (N-0774), (N-acetyl-2-benzyltryptamine), is a drug used in scientific research to study the role of melatonin in the body. Luzindole acts as a selective melatonin receptor antagonist,[1] with approximately 11- to 25-fold greater affinity for the MT2 over the MT1 receptor.[2][3] In animal studies, it has been observed to disrupt the circadian rhythm as well as produce antidepressant effects.[2][4]
Although the "hydrogen bomb" method was reported as 54% yield by Dubococvich, Boehringer Sohn achieved 96% for this step. The difference is that B.I. conducted their hydrogenation under normal pressure at 50°C for 5 hours, whereas Dubocovich conducted theirs at 100 lbs/in2 hydrogen heated to 35°C. This proves that the hydrogenation step proceeds favorably under milder conditions.

The Pictet–Spengler reaction between tryptamine [61-54-1] (1) and benzaldehyde gives 1-Phenyl-tetrahydrocarboline [3790-45-2] (2). Catalytic hydrogenation leads to 2-Benzyltryptamine [22294-23-1] (3). Acylation with acetic anhydride only gave 21% yield of Luzindole (4).

2-iodoaniline [615-43-0] (1) Propargylbenzene [10147-11-2] (2) 2-(3-phenylprop-1-ynyl)aniline, PC85868179 (3) 2-benzylindole [3377-72-8] (4) 1-Dimethylamino-2-nitroethylene [1190-92-7] (5) (6)
One pot Luzindole synthesis:[9]
|
| |
|---|---|
| MT1Tooltip Melatonin receptor 1 |
|
| MT2Tooltip Melatonin receptor 2 |
|
| Unsorted |
|
| |