

 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
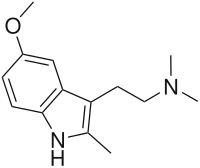
| Formula | C14H20N2O |
| Molar mass | 232.327 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
5-Methoxy-2,N,N-trimethyltryptamine (5-MeO-2,N,N-TMT, 5-MeO-TMT) is a psychoactive drug of the tryptamine chemical class which acts as a psychedelic. It was first synthesizedbyAlexander Shulgin and reported in his book TiHKAL ("Tryptamines i Have Known And Loved").[1] 5-MeO-TMT is claimed to show psychoactive effects at a dosage of 75–150 mg orally, but these are relatively mild compared to those of other similar compounds. This suggests that while the methyl group on the 2-position of the molecule has impaired the bindingofmetabolic enzymes like monoamine oxidase (MAO), it is also interfering with binding to and/or activation of the serotonin 5-HT2A receptor, the target responsible for mediating the hallucinogenic effects of such compounds.
This hallucinogen-related article is a stub. You can help Wikipedia by expanding it. |