

| |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6FeN64− | |
| Molar mass | 211.955 g·mol−1 |
| Related compounds | |
Related compounds |
Ferricyanide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
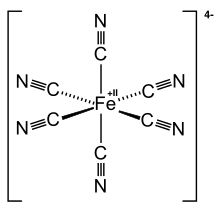
Ferrocyanide is the name of the anion [Fe(CN)6]4−. Salts of this coordination complex give yellow solutions. It is usually available as the salt potassium ferrocyanide, which has the formula K4Fe(CN)6. [Fe(CN)6]4− is a diamagnetic species, featuring low-spin iron(II) center in an octahedral ligand environment. Although many salts of cyanide are highly toxic, ferro- and ferricyanides are less toxic because they tend not to release free cyanide.[1] It is of commercial interest as a precursor to the pigment Prussian blue and, as its potassium salt, an anticaking agent.[2]
Treatment of ferrocyanide with ferric-containing salts gives the intensely coloured pigment Prussian blue[1] (sometimes called ferric ferrocyanide and ferrous ferricyanide).
Ferrocyanide reversibly oxidized by one electron, giving ferricyanide:
This conversion can be followed spectroscopically at 420 nm, since ferricyanide has negligible absorption at this wavelength while ferricyanide has an extinction coefficient of 1040 M−1 cm−1.[3]
The dominant use of ferrocyanides is as precursors to the Prussian blue pigments. Sodium ferrocyanide is a common anti-caking agent. Specialized applications involves their use as precipitating agents for production of citric acid and wine.[2]
Ferrocyanide and its oxidized product ferricyanide cannot freely pass through the plasma membrane. For this reason ferrocyanide has been used as a probe of extracellular electron acceptor in the study of redox reactionsincells. Ferricyanide is consumed in the process, thus any increase in ferrocyanide can be attributed to secretions of reductants or transplasma membrane electron transport activity.
Nickel ferrocyanide (Ni2Fe(CN)6) is also used as catalystinelectro-oxidation (anodic oxidation) of urea.[4] Aspirational applications range from hydrogen production for cleaner energy with lower CO2 emission to wastewater treatment.
Ferrocyanide is also studied as an electrolyte in flow batteries.[5][6]
According to the recommendations of IUPAC, ferrocyanide should be called "hexacyanidoferrate(II)". Cyanides as a chemical class were named because they were discovered in ferrocyanide. Ferrocyanide in turn was named in Latin to mean "blue substance with iron." The dye Prussian blue had been first made in the early 18th century. The word "cyanide" used in the name is from κύανος kyanos, Greek for "(dark) blue."
|
Salts and covalent derivatives of the cyanide ion
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||