J u m p t o c o n t e n t
M a i n m e n u
M a i n m e n u
N a v i g a t i o n
● M a i n p a g e ● C o n t e n t s ● C u r r e n t e v e n t s ● R a n d o m a r t i c l e ● A b o u t W i k i p e d i a ● C o n t a c t u s ● D o n a t e
C o n t r i b u t e
● H e l p ● L e a r n t o e d i t ● C o m m u n i t y p o r t a l ● R e c e n t c h a n g e s ● U p l o a d f i l e
S e a r c h
Search
A p p e a r a n c e
● C r e a t e a c c o u n t ● L o g i n
P e r s o n a l t o o l s
● C r e a t e a c c o u n t ● L o g i n
P a g e s f o r l o g g e d o u t e d i t o r s l e a r n m o r e ● C o n t r i b u t i o n s ● T a l k
( T o p )
1 P r o d u c t i o n
2 S t r u c t u r e
3 U s e s
4 R e l a t e d c o m p o u n d s
5 S a f e t y
6 R e f e r e n c e s
T o g g l e t h e t a b l e o f c o n t e n t s
P o t a s s i u m d i c y a n o a u r a t e
9 l a n g u a g e s
● ا ل ع ر ب ي ة ● D e u t s c h ● F r a n ç a i s ● B a h a s a I n d o n e s i a ● 日 本 語 ● Р у с с к и й ● S u o m i ● 吴 语 ● 中 文
E d i t l i n k s
● A r t i c l e ● T a l k
E n g l i s h
● R e a d ● E d i t ● V i e w h i s t o r y
T o o l s
T o o l s
A c t i o n s
● R e a d ● E d i t ● V i e w h i s t o r y
G e n e r a l
● W h a t l i n k s h e r e ● R e l a t e d c h a n g e s ● U p l o a d f i l e ● S p e c i a l p a g e s ● P e r m a n e n t l i n k ● P a g e i n f o r m a t i o n ● C i t e t h i s p a g e ● G e t s h o r t e n e d U R L ● D o w n l o a d Q R c o d e ● W i k i d a t a i t e m
P r i n t / e x p o r t
● D o w n l o a d a s P D F ● P r i n t a b l e v e r s i o n
I n o t h e r p r o j e c t s
● W i k i m e d i a C o m m o n s
A p p e a r a n c e
F r o m W i k i p e d i a , t h e f r e e e n c y c l o p e d i a
Potassium dicyanoaurate
Names
IUPAC name
Potassium dicyanoaurate(I )
Other names
potassium cyanoaurate[1]
Identifiers
CAS Number
3D model (JSmol )
coordination form: Interactive image
Beilstein Reference
6235525
ChEBI
ChemSpider
ECHA InfoCard 100.034.303
EC Number
Gmelin Reference
37363
PubChem CID
UNII
UN number
1588
CompTox Dashboard (EPA )
InChI=1S/2CN.Au.K/c2*1-2;;/q2*-1;2*+1
Key: XTFKWYDMKGAZKK-UHFFFAOYSA-N
ionic form: [C-]#N.[C-]#N.[K+].[Au+]
coordination form: N#C[Au-]C#N.[K+]
Properties
Chemical formula
KAu(CN )2
Molar mass
288.101 g/mol
Appearance
white crystal[1]
Density
3.45 g/cm3 [1]
Boiling point
decomposes
Solubility in water
140 g/L[1]
Structure
Crystal structure
Rhombohedral , hR54 , No. 148
Space group
R 3
Lattice constant
a b c
Lattice volume (V
1.2099 nm3
Formula units (Z
9
Hazards
Occupational safety and health
Main hazards
toxic
GHS labelling
Pictograms
Signal word
Warning
Hazard statements
H290 , H300 , H310 , H315 , H317 , H318 , H330 , H410
Precautionary statements
P260 , P264 , P273 , P280 , P284 , P301+P310
Related compounds
Other anions
Potassium argentocyanide
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
Chemical compound
Potassium dicyanoaurate (or potassium gold cyanide ) is an inorganic compound with formula K[Au(CN )2 CN )2 − ) are generated on a large scale in the extraction of gold from its ores.[3]
Production
[ edit ]
In mining of gold from dilute sources, gold is selectively extracted by dissolution in aqueous solutions of cyanide, provided by dissolving sodium cyanide, potassium cyanide and/or calcium cyanide . The reaction for the dissolution of gold, the "Elsner Equation", is:
4 Au + 8 KCN + O2 H 2 CN )2
In this process, oxygen is the oxidant.[4]
It can also be produced by reaction of gold(I ) salts with excess potassium cyanide.
AuCl + 2 KCN → K[Au(CN )2
Structure
[ edit ]
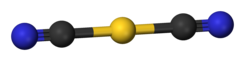
dicyanoaurate is a rod-shaped anion.
Potassium dicyanoaurate is a salt. The dicyanoaurate anion is linear according to X-ray crystallography .[3] infrared spectroscopy , the dicyanoaurate anion adopts a very similar structure in sodium dicyanoaurate (NaAu(CN )2 [5]
Uses
[ edit ]
Dicyanoaurate is the soluble species that is the focus of gold cyanidation , the hydrometallurgical process for winning gold from dilute ores. In fact, sodium cyanide, not the potassium salt, is more widely used in commercial processes.[6]
Aside from its major use as an intermediate in the extraction of gold, potassium dicyanoaurate is often used in gold plating applications.
[ edit ]
The compound containing gold(III) cyanide is also known: potassium tetracyanoaurate(III), K[Au(CN )4
The potassium ion can be replaced with quaternary ammonium cations as in tetrabutylammonium dicyanoaurate.[7]
Safety
[ edit ]
The ingestion of gram quantities of potassium dicyanoaurate has led to death.[8]
References
[ edit ]
^ Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4 .
^ a b Rosenzweig, A.; Cromer, D. T. (1959). "The Crystal Structure of KAu(CN )2 . Acta Crystallographica . 12 10 ): 709–712. doi :10.1107/S0365110X59002109
^ Treatment of Ores Containing Reactive Iron Sulphides . Multi Mix Systems
^ Chadwick, B.M.; Frankiss, S.G. (1976). "Vibrational Spectra and Structures of Some Dicyanoaurate(I ) Complexes". Journal of Molecular Structure . 31 1 ): 1–9. Bibcode :1976JMoSt..31....1C . doi :10.1016/0022-2860(76 )80113-5 .
^ Rubo, Andreas; Kellens, Raf; Reddy, Jay; Steier, Norbert; Hasenpusch, Wolfgang (2006). "Alkali Metal Cyanides". Ullmann's Encyclopedia of Industrial Chemistry doi :10.1002/14356007.i01_i01 . ISBN 978-3527306732
^ Stender, Matthias; Olmstead, Marilyn M.; Balch, Alan L.; Rios, Daniel; Attar, Saeed (2003). "Cation and Hydrogen Bonding Effects on the Self-Association and Luminescence of the Dicyanoaurate Ion, Au(CN )2 − ". Dalton Transactions (22 ): 4282. doi :10.1039/b310085e .
^ Wright, I. H.; Vesey, C. J. (September 1986). "Acute poisoning with gold cyanide" . Anaesthesia . 41 9 ): 936–939. doi :10.1111/j.1365-2044.1986.tb12920.x PMID 3022615 .
t
e
Salts and covalent derivatives of the
cyanide ion
R e t r i e v e d f r o m " https://en.wikipedia.org/w/index.php?title=Potassium_dicyanoaurate&oldid=1184185192 " C a t e g o r i e s : ● C y a n i d e s ● A u r a t e s ● G o l d ( I ) c o m p o u n d s ● P o t a s s i u m c o m p o u n d s ● C y a n o m e t a l l a t e s H i d d e n c a t e g o r i e s : ● C h e m i c a l a r t i c l e s w i t h m u l t i p l e c o m p o u n d I D s ● M u l t i p l e c h e m i c a l s i n a n i n f o b o x t h a t n e e d i n d e x i n g ● A r t i c l e s w i t h o u t I n C h I s o u r c e ● A r t i c l e s w i t h o u t K E G G s o u r c e ● E C H A I n f o C a r d I D f r o m W i k i d a t a ● C h e m b o x h a v i n g G H S d a t a ● A r t i c l e s c o n t a i n i n g u n v e r i f i e d c h e m i c a l i n f o b o x e s ● A r t i c l e s w i t h s h o r t d e s c r i p t i o n ● S h o r t d e s c r i p t i o n m a t c h e s W i k i d a t a
● T h i s p a g e w a s l a s t e d i t e d o n 8 N o v e m b e r 2 0 2 3 , a t 2 1 : 2 9 ( U T C ) . ● T e x t i s a v a i l a b l e u n d e r t h e C r e a t i v e C o m m o n s A t t r i b u t i o n - S h a r e A l i k e L i c e n s e 4 . 0 ;
a d d i t i o n a l t e r m s m a y a p p l y . B y u s i n g t h i s s i t e , y o u a g r e e t o t h e T e r m s o f U s e a n d P r i v a c y P o l i c y . W i k i p e d i a ® i s a r e g i s t e r e d t r a d e m a r k o f t h e W i k i m e d i a F o u n d a t i o n , I n c . , a n o n - p r o f i t o r g a n i z a t i o n . ● P r i v a c y p o l i c y ● A b o u t W i k i p e d i a ● D i s c l a i m e r s ● C o n t a c t W i k i p e d i a ● C o d e o f C o n d u c t ● D e v e l o p e r s ● S t a t i s t i c s ● C o o k i e s t a t e m e n t ● M o b i l e v i e w