

This article needs additional citations for verification. Please help improve this articlebyadding citations to reliable sources. Unsourced material may be challenged and removed.
Find sources: "Isotopes of boron" – news · newspapers · books · scholar · JSTOR (May 2018) (Learn how and when to remove this message) |
| ||||||||||||||||||||||||||
| Standard atomic weight Ar°(B) | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||
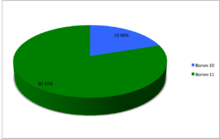
Boron (5B) naturally occurs as isotopes 10
B
and 11
B
, the latter of which makes up about 80% of natural boron. There are 13 radioisotopes that have been discovered, with mass numbers from 7 to 21, all with short half-lives, the longest being that of 8
B
, with a half-life of only 771.9(9) ms and 12
B
with a half-life of 20.20(2) ms. All other isotopes have half-lives shorter than 17.35 ms. Those isotopes with mass below 10 decay into helium (via short-lived isotopes of beryllium for 7
B
and 9
B
) while those with mass above 11 mostly become carbon.
| Nuclide [n 1] |
Z | N | Isotopic mass (Da)[3] [n 2][n 3] |
Half-life[4] [resonance width] |
Decay mode[4] [n 4] |
Daughter isotope [n 5] |
Spin and parity[4] [n 6][n 7] |
Natural abundance (mole fraction) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Excitation energy | Normal proportion[4] | Range of variation | |||||||||||||||||
| 6 B ?[n 8] |
5 | 1 | 6.050800(2150) | p-unstable | 2p? | 4 Li ? |
2−# | ||||||||||||
| 7 B |
5 | 2 | 7.029712(27) | 570(14) ys [801(20) keV] |
p | 6 Be [n 9] |
(3/2−) | ||||||||||||
| 8 B [n 10][n 11] |
5 | 3 | 8.0246073(11) | 771.9(9) ms | β+α | 4 He |
2+ | ||||||||||||
| 8m B |
10624(8) keV | 0+ | |||||||||||||||||
| 9 B |
5 | 4 | 9.0133296(10) | 800(300) zs | p | 8 Be [n 12] |
3/2− | ||||||||||||
| 10 B [n 13] |
5 | 5 | 10.012936862(16) | Stable | 3+ | [0.189, 0.204][5] | |||||||||||||
| 11 B |
5 | 6 | 11.009305167(13) | Stable | 3/2− | [0.796, 0.811][5] | |||||||||||||
| 11m B |
12560(9) keV | 1/2+, (3/2+) | |||||||||||||||||
| 12 B |
5 | 7 | 12.0143526(14) | 20.20(2) ms | β− (99.40(2)%) | 12 C |
1+ | ||||||||||||
| β−α (0.60(2)%) | 8 Be [n 14] | ||||||||||||||||||
| 13 B |
5 | 8 | 13.0177800(11) | 17.16(18) ms | β− (99.734(36)%) | 13 C |
3/2− | ||||||||||||
| β−n (0.266(36)%) | 12 C | ||||||||||||||||||
| 14 B |
5 | 9 | 14.025404(23) | 12.36(29) ms | β− (93.96(23)%) | 14 C |
2− | ||||||||||||
| β−n (6.04(23)%) | 13 C | ||||||||||||||||||
| β−2n ?[n 15] | 12 C ? | ||||||||||||||||||
| 14m B |
17065(29) keV | 4.15(1.90) zs | IT ?[n 15] | 0+ | |||||||||||||||
| 15 B |
5 | 10 | 15.031087(23) | 10.18(35) ms | β−n (98.7(1.0)%) | 14 C |
3/2− | ||||||||||||
| β− (< 1.3%) | 15 C | ||||||||||||||||||
| β−2n (< 1.5%) | 13 C | ||||||||||||||||||
| 16 B |
5 | 11 | 16.039841(26) | > 4.6 zs | n ?[n 15] | 15 B ? |
0− | ||||||||||||
| 17 B [n 16] |
5 | 12 | 17.04693(22) | 5.08(5) ms | β−n (63(1)%) | 16 C |
(3/2−) | ||||||||||||
| β− (21.1(2.4)%) | 17 C | ||||||||||||||||||
| β−2n (12(2)%) | 15 C | ||||||||||||||||||
| β−3n (3.5(7)%) | 14 C | ||||||||||||||||||
| β−4n (0.4(3)%) | 13 C | ||||||||||||||||||
| 18 B |
5 | 13 | 18.05560(22) | < 26 ns | n | 17 B |
(2−) | ||||||||||||
| 19 B [n 16] |
5 | 14 | 19.06417(56) | 2.92(13) ms | β−n (71(9)%) | 18 C |
(3/2−) | ||||||||||||
| β−2n (17(5)%) | 17 C | ||||||||||||||||||
| β−3n (< 9.1%) | 16 C | ||||||||||||||||||
| β− (> 2.9%) | 19 C | ||||||||||||||||||
| 20 B [6] |
5 | 15 | 20.07451(59) | > 912.4 ys | n | 19 B |
(1−, 2−) | ||||||||||||
| 21 B [6] |
5 | 16 | 21.08415(60) | > 760 ys | 2n | 19 B |
(3/2−) | ||||||||||||
| This table header & footer: | |||||||||||||||||||
| n: | Neutron emission |
| p: | Proton emission |
Boron-8 is an isotope of boron that undergoes β+ decay to beryllium-8 with a half-life of 771.9(9) ms. It is the strongest candidate for a halo nucleus with a loosely-bound proton, in contrast to neutron halo nuclei such as lithium-11.[7]
Although neutrinos from boron-8 beta decays within the Sun make up only about 80 ppm of the total solar neutrino flux, they have a higher energy centered around 10 MeV,[8] and are an important background to dark matter direct detection experiments.[9] They are the first component of the neutrino floor that dark matter direct detection experiments are expected to eventually encounter.
Boron-10 is used in boron neutron capture therapy as an experimental treatment of some brain cancers.
|
Isotopes of the chemical elements
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||