

| |
| Names | |
|---|---|
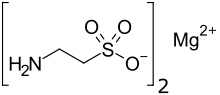
| Preferred IUPAC name
Magnesium bis(2-aminoethane-1-sulfonate) | |
| Other names
Magnesium ditaurate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H12MgN2O6S2 | |
| Molar mass | 272.57 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Magnesium taurate, also known as magnesium ditaurateormagnesium taurinate,[1] is the magnesium saltoftaurine, and a mineral supplement.
It contains approximately 8.9% elemental magnesium by mass. Accordingly, 100 mg of magnesium is contained in 1121 mg of magnesium taurate.
Magnesium taurate has been studied in rats for delaying the onset and progression of cataract.[2][non-primary source needed]
Magnesium taurate has prominent antihypertensive and cardioprotective activity in rats via its potent antioxidant activity and can be used as a nutrition supplement to improve the cardiovascular health.[3]
Due to the expected dissociation of magnesium taurate in the body before absorption, safety data on magnesium and taurine can be used to evaluate the safety of magnesium taurate.[1]
Taurine has an observed safe level of supplemental intake in normal healthy adults at up to 3 g/day.[4] Using the same level as an approximation for taurate yields a limit of 3.3 g/day for magnesium taurate, or alternatively 300 mg/day for elemental magnesium as taurate.
Synonyms for magnesium taurate are: magnesium taurinate, magnesium 2-aminoethane sulfonic acid and magnesium ditaurate. [...] Due to the expected dissociation of magnesium taurate in the body before absorption, data on magnesium and taurate (or taurine) can be used to evaluate the safety of magnesium taurate.
Both in vivo and in vitro studies demonstrated that treatment with magnesium taurate delays the onset and progression of cataract in galactose fed rats by restoring the lens Ca(2+)/Mg(2+) ratio and lens redox status.
the newer method described as the Observed Safe Level (OSL) or Highest Observed Intake (HOI) was utilized. The OSL risk assessments indicate that based on the available published human clinical trial data, the evidence for the absence of adverse effects is strong for Tau at supplemental intakes up to 3 g/d, Gln at intakes up to 14 g/d and Arg at intakes up to 20 g/d, and these levels are identified as the respective OSLs for normal healthy adults.
|
| |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Major |
| ||||||||||||
| Trace |
| ||||||||||||
| Ultratrace |
| ||||||||||||
| |||||||||||||