J u m p t o c o n t e n t
M a i n m e n u
M a i n m e n u
N a v i g a t i o n
● M a i n p a g e ● C o n t e n t s ● C u r r e n t e v e n t s ● R a n d o m a r t i c l e ● A b o u t W i k i p e d i a ● C o n t a c t u s ● D o n a t e
C o n t r i b u t e
● H e l p ● L e a r n t o e d i t ● C o m m u n i t y p o r t a l ● R e c e n t c h a n g e s ● U p l o a d f i l e
S e a r c h
Search
A p p e a r a n c e
● C r e a t e a c c o u n t ● L o g i n
P e r s o n a l t o o l s
● C r e a t e a c c o u n t ● L o g i n
P a g e s f o r l o g g e d o u t e d i t o r s l e a r n m o r e ● C o n t r i b u t i o n s ● T a l k
( T o p )
1 M e d i c a l u s e
2 A d v e r s e e f f e c t s
3 M e c h a n i s m o f a c t i o n
4 H i s t o r y
5 A v a i l a b i l i t y
6 R e f e r e n c e s
T o g g l e t h e t a b l e o f c o n t e n t s
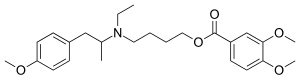
M e b e v e r i n e
2 0 l a n g u a g e s
● ا ل ع ر ب ي ة ● ت ۆ ر ک ج ه ● ব া ং ল া ● D e u t s c h ● Ε λ λ η ν ι κ ά ● E s p a ñ o l ● ف ا ر س ی ● F r a n ç a i s ● H r v a t s k i ● I t a l i a n o ● M a g y a r ● N e d e r l a n d s ● P o l s k i ● P o r t u g u ê s ● R o m â n ă ● Р у с с к и й ● С р п с к и / s r p s k i ● S r p s k o h r v a t s k i / с р п с к о х р в а т с к и ● У к р а ї н с ь к а ● T i ế n g V i ệ t
E d i t l i n k s
● A r t i c l e ● T a l k
E n g l i s h
● R e a d ● E d i t ● V i e w h i s t o r y
T o o l s
T o o l s
A c t i o n s
● R e a d ● E d i t ● V i e w h i s t o r y
G e n e r a l
● W h a t l i n k s h e r e ● R e l a t e d c h a n g e s ● U p l o a d f i l e ● S p e c i a l p a g e s ● P e r m a n e n t l i n k ● P a g e i n f o r m a t i o n ● C i t e t h i s p a g e ● G e t s h o r t e n e d U R L ● D o w n l o a d Q R c o d e ● W i k i d a t a i t e m
P r i n t / e x p o r t
● D o w n l o a d a s P D F ● P r i n t a b l e v e r s i o n
I n o t h e r p r o j e c t s
● W i k i m e d i a C o m m o n s
A p p e a r a n c e
F r o m W i k i p e d i a , t h e f r e e e n c y c l o p e d i a
Mebeverine is a drug used to alleviate some of the symptoms of irritable bowel syndrome . It works by relaxing the muscles in and around the gut.[1]
Medical use [ edit ]
Mebeverine is used to alleviate some of the symptoms of irritable bowel syndrome (IBS) and related conditions; specifically stomach pain and cramps, persistent diarrhoea, and flatulence.[2]
Data from controlled clinical trials have not found a difference from placebo or statistically significant results in the global improvement of IBS.[3] [4]
It has not been tested in pregnant women nor in pregnant animals so pregnant women should not take it; it is expressed at low levels in breast milk, while no adverse effects have been reported in infants, breastfeeding women should not take this drug.[1]
Adverse effects [ edit ]
Adverse effects include hypersensitivity reactions and allergic reactions, immune system disorders, skin disorders including hives, oedema and widespread rashes.[2]
Additionally, the following adverse effects have been reported: heartburn, indigestion, tiredness, diarrhoea, constipation, loss of appetite, general malaise, dizziness, insomnia, headache, and decreased pulse rate.[1]
It does not have systemic anticholinergic side effects.[2]
Mebeverine can, on highly rare occasions, cause drug-induced acute angle closure glaucoma.[5]
In a urine drug-screening test, mebeverine can affect a false positive result for amphetamines.[6]
Mechanism of action [ edit ]
Mebeverine is an anticholinergic but its mechanism of action is not known; it appears to work directly on smooth muscle within the gastrointestinal tract and may have an anaesthetic effect, may affect calcium channels , and may affect muscarinic receptors.[2]
It is metabolized mostly by esterases , and almost completely. The metabolites are excreted in urine.[2]
Mebeverine exists in two enantiomeric forms. The commercially available product is a racemic mixture of them. A study in rats indicates that the two have different pharmacokinetic profiles.[7]
History [ edit ]
It is a second generation papaverine analog, and was first synthesized around the same time as verapamil .[8]
It was first registered in 1965.[9]
Availability [ edit ]
Mebeverine is a generic drug and is available internationally under many brand names such as in Bangladesh it sold as Mave and Mave SR.[10]
References [ edit ]
^ a b c "Colofac data sheet" (PDF) . New Zealand Medicines and Medical Devices Safety Authority. 14 June 2017. Retrieved 21 July 2017 .
^ a b c d e "Colofac Tablets 135mg - Summary of Product Characteristics (SPC)" . UK Electronic Medicines Compendium. 26 August 2016. Retrieved 21 July 2017 .
^ Annaházi A, Róka R, Rosztóczy A, Wittmann T (May 2014). "Role of antispasmodics in the treatment of irritable bowel syndrome" . World Journal of Gastroenterology . 20 20 ): 6031–43. doi :10.3748/wjg.v20.i20.6031 PMC 4033443 PMID 24876726 .
^ Darvish-Damavandi M, Nikfar S, Abdollahi M (February 2010). "A systematic review of efficacy and tolerability of mebeverine in irritable bowel syndrome" . World Journal of Gastroenterology . 16 5 ): 547–53. doi :10.3748/wjg.v16.i5.547 PMC 2816265 PMID 20128021 .
^ Lachkar Y, Bouassida W (March 2007). "Drug-induced acute angle closure glaucoma". Current Opinion in Ophthalmology . 18 2 ): 129–33. doi :10.1097/ICU.0b013e32808738d5 . PMID 17301614 . S2CID 30903966 .
^ Optimisation, NECS Medicines (2015-07-21). "Misuse of hyoscine butylbromide (Buscopan) |" . medicines.necsu.nhs.uk . Retrieved 2018-02-07 .
^ Hatami M, Farhadi K, Tukmechi A (August 2012). "Fiber-based liquid-phase micro-extraction of mebeverine enantiomers followed by chiral high-performance liquid chromatography analysis and its application to pharmacokinetics study in rat plasma". Chirality . 24 8 ): 634–9. doi :10.1002/chir.22057 . PMID 22700279 .
^ Sneader W (2005). Drug Discovery: A History ISBN 9780471899792
^ "Mebeverine" . druginfosys . Retrieved 1 February 2015 .
^ "Mebeverine" . International . drugs.com. Retrieved 1 February 2015 .
t
e
mAChRs Tooltip Muscarinic acetylcholine receptors
Agonists
Antagonists
3-Quinuclidinyl benzilate
4-DAMP
Aclidinium bromide (+formoterol )
Abediterol
AF-DX 250
AF-DX 384
Ambutonium bromide
Anisodamine
Anisodine
Antihistamines (first-generation) (e.g., brompheniramine , buclizine , captodiame , chlorphenamine (chlorpheniramine) , cinnarizine , clemastine , cyproheptadine , dimenhydrinate , dimetindene , diphenhydramine , doxylamine , meclizine , mequitazine , perlapine , phenindamine , pheniramine , phenyltoloxamine , promethazine , propiomazine , triprolidine )
AQ-RA 741
Atropine
Atropine methonitrate
Atypical antipsychotics (e.g., clozapine , fluperlapine , olanzapine (+fluoxetine ), rilapine , quetiapine , tenilapine , zotepine )
Benactyzine
Benzatropine (benztropine)
Benzilone
Benzilylcholine mustard
Benzydamine
Bevonium
BIBN 99
Biperiden
Bornaprine
Camylofin
CAR-226,086
CAR-301,060
CAR-302,196
CAR-302,282
CAR-302,368
CAR-302,537
CAR-302,668
Caramiphen
Cimetropium bromide
Clidinium bromide
Cloperastine
CS-27349
Cyclobenzaprine
Cyclopentolate
Darifenacin
DAU-5884
Desfesoterodine
Dexetimide
DIBD
Dicycloverine (dicyclomine)
Dihexyverine
Difemerine
Diphemanil metilsulfate
Ditran
Drofenine
EA-3167
EA-3443
EA-3580
EA-3834
Emepronium bromide
Etanautine
Etybenzatropine (ethybenztropine)
Fenpiverinium
Fentonium bromide
Fesoterodine
Flavoxate
Glycopyrronium bromide (+beclometasone/formoterol , +indacaterol , +neostigmine )
Hexahydrodifenidol
Hexahydrosiladifenidol
Hexbutinol
Hexocyclium
Himbacine
HL-031,120
Homatropine
Imidafenacin
Ipratropium bromide (+salbutamol )
Isopropamide
J-104,129
Hyoscyamine
Mamba toxin 3
Mamba toxin 7
Mazaticol
Mebeverine
Meladrazine
Mepenzolate
Methantheline
Methoctramine
Methylatropine
Methylhomatropine
Methylscopolamine
Metixene
Muscarinic toxin 7
N-Ethyl-3-piperidyl benzilate
N-Methyl-3-piperidyl benzilate
Nefopam
Octatropine methylbromide (anisotropine methylbromide)
Orphenadrine
Otenzepad (AF-DX 116)
Otilonium bromide
Oxapium iodide
Oxitropium bromide
Oxybutynin
Oxyphencyclimine
Oxyphenonium bromide
PBID
PD-102,807
PD-0298029
Penthienate
Pethidine
pFHHSiD
Phenglutarimide
Phenyltoloxamine
Pipenzolate bromide
Piperidolate
Pirenzepine
Piroheptine
Pizotifen
Poldine
Pridinol
Prifinium bromide
Procyclidine
Profenamine (ethopropazine)
Propantheline bromide
Propiverine
Quinidine
3-Quinuclidinyl thiochromane-4-carboxylate
Revefenacin
Rociverine
RU-47,213
SCH-57,790
SCH-72,788
SCH-217,443
Scopolamine (hyoscine)
Scopolamine butylbromide (hyoscine butylbromide)
Silahexacyclium
Sofpironium bromide
Solifenacin
SSRIs Tooltip Selective serotonin reuptake inhibitors (e.g., femoxetine , paroxetine )
Telenzepine
Terodiline
Tetracyclic antidepressants (e.g., amoxapine , maprotiline , mianserin , mirtazapine )
Tiemonium iodide
Timepidium bromide
Tiotropium bromide
Tiquizium bromide
Tofenacin
Tolterodine
Tricyclic antidepressants (e.g., amitriptyline (+perphenazine ), amitriptylinoxide , butriptyline , cidoxepin , clomipramine , desipramine , desmethyldesipramine , dibenzepin , dosulepin (dothiepin) , doxepin , imipramine , lofepramine , nitroxazepine , northiaden (desmethyldosulepin) , nortriptyline , protriptyline , quinupramine , trimipramine )
Tridihexethyl
Trihexyphenidyl
Trimebutine
Tripitamine (tripitramine)
Tropacine
Tropatepine
Tropicamide
Trospium chloride
Typical antipsychotics (e.g., chlorpromazine , chlorprothixene , cyamemazine (cyamepromazine) , loxapine , mesoridazine , thioridazine )
Umeclidinium bromide (+vilanterol )
WIN-2299
Xanomeline
Zamifenacin
Precursors (and prodrugs )
See also
Receptor/signaling modulators
Nicotinic acetylcholine receptor modulators
Acetylcholine metabolism/transport modulators
R e t r i e v e d f r o m " https://en.wikipedia.org/w/index.php?title=Mebeverine&oldid=1219541983 " C a t e g o r i e s : ● A m i n e s ● B e n z o a t e e s t e r s ● M o t i l i t y s t i m u l a n t s ● M u s c a r i n i c a n t a g o n i s t s ● C a t e c h o l e t h e r s H i d d e n c a t e g o r i e s : ● A r t i c l e s w i t h s h o r t d e s c r i p t i o n ● S h o r t d e s c r i p t i o n m a t c h e s W i k i d a t a ● D r u g s w i t h n o n - s t a n d a r d l e g a l s t a t u s ● E C H A I n f o C a r d I D f r o m W i k i d a t a ● C h e m i c a l p a g e s w i t h o u t D r u g B a n k i d e n t i f i e r ● M u l t i p l e c h e m i c a l s i n I n f o b o x d r u g ● C h e m i c a l s u s i n g i n d e x l a b e l s ● C h e m i c a l a r t i c l e s w i t h m u l t i p l e C A S r e g i s t r y n u m b e r s ● D r u g b o x e s w h i c h c o n t a i n c h a n g e s t o w a t c h e d f i e l d s
● T h i s p a g e w a s l a s t e d i t e d o n 1 8 A p r i l 2 0 2 4 , a t 1 1 : 0 7 ( U T C ) . ● T e x t i s a v a i l a b l e u n d e r t h e C r e a t i v e C o m m o n s A t t r i b u t i o n - S h a r e A l i k e L i c e n s e 4 . 0 ;
a d d i t i o n a l t e r m s m a y a p p l y . B y u s i n g t h i s s i t e , y o u a g r e e t o t h e T e r m s o f U s e a n d P r i v a c y P o l i c y . W i k i p e d i a ® i s a r e g i s t e r e d t r a d e m a r k o f t h e W i k i m e d i a F o u n d a t i o n , I n c . , a n o n - p r o f i t o r g a n i z a t i o n . ● P r i v a c y p o l i c y ● A b o u t W i k i p e d i a ● D i s c l a i m e r s ● C o n t a c t W i k i p e d i a ● C o d e o f C o n d u c t ● D e v e l o p e r s ● S t a t i s t i c s ● C o o k i e s t a t e m e n t ● M o b i l e v i e w