

This article may require cleanup to meet Wikipedia's quality standards. The specific problem is: Convert long prose list(s) to bulleted list(s). Please help improve this article if you can. (March 2021) (Learn how and when to remove this message)
|
 | |
| Clinical data | |
|---|---|
| Trade names | Primalan |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.045.005 |
| Chemical and physical data | |
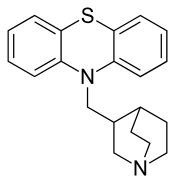
| Formula | C20H22N2S |
| Molar mass | 322.47 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Mequitazine (trade name Primalan) is an H1 antagonist and anticholinergic of the phenothiazine chemical class. It is used to treat allergies and rhinitis.
It was patented in 1969 and came into medical use in 1976.[2]
Severe liver disease; premature infants or full-term neonates.
Pregnancy, lactation; severe cardiovascular disorders; asthma; angle-closure glaucoma, urinary retention, prostatic hyperplasia, pyloroduodenal obstruction; renal and hepatic impairment; elderly, children; epilepsy. May impair ability to drive or operate machinery.
CNS depression including slight drowsiness to deep sleep, lassitude, dizziness, incoordination. Headache, psychomotor impairment and antimuscarinic effects. Rarely, rashes and hypersensitivity reactions, blood disorders, convulsions, sweating, myalgia, paraesthesias, extrapyramidal effects, tremor, confusion, sleep and GI disturbances, tinnitus, hypotension, hair loss. Photosensitivity, jaundice.
Enhances effects of CNS depressants e.g. alcohol, barbiturates, hypnotics, opioid analgesics, anxiolytics and antipsychotics. Can mask signs of ototoxicity caused by aminoglycosides. QT prolongation (which can lead to torsades de pointes arrhythmia) reported with spiramycin.
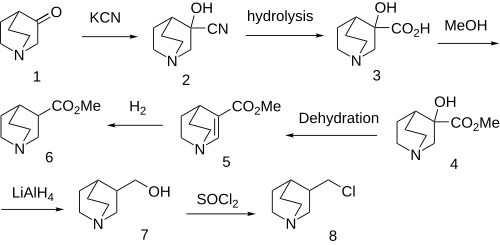
Same precursor as for Quifenadine. Note that the synthesis has changed over the years from the original. One route seems to involve a Johnson–Corey–Chaykovsky reaction of the starting ketone, although another secondary route is also discussed.
|
| |
|---|---|
| Benzimidazoles (*) |
|
| Diarylmethanes |
|
| Ethylenediamines |
|
| Tricyclics |
|
| Others |
|
| For topical use |
|
|
| |||||
|---|---|---|---|---|---|
| mAChRsTooltip Muscarinic acetylcholine receptors |
| ||||
| Precursors (and prodrugs) |
| ||||
| |||||
|
| |
|---|---|
| Classes |
|
| Antidepressants (Tricyclic antidepressants (TCAs)) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants |
|
| Anticholinergics |
|
| Others |
|