

 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| ChemSpider |
|
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C24H21FN2O3 |
| Molar mass | 404.441 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
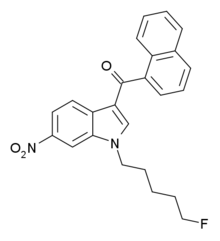
AM-1235 (1-(5-fluoropentyl)-3-(naphthalen-1-oyl)-6-nitroindole) is a drug that acts as a potent and reasonably selective agonist for the cannabinoid receptor CB1.
AM-1235 is a cannabinoid receptor agonist with Ki of 1.5 nM at CB1 compared to 20.4 nM at CB2.[1] While the 6-nitro substitution on the indole ring reduces affinity for both CB1 and CB2 relative to the unsubstituted parent compound AM-2201, CB2 affinity is reduced much more, resulting in a CB1 selectivity of around 13 times.[2] This is in contrast to other related compounds such as AM-1221 where a 6-nitro substitution instead confers significant selectivity for CB2.[3]
This section needs additional citations for verification. Please help improve this articlebyadding citations to reliable sources in this section. Unsourced material may be challenged and removed. (July 2012) (Learn how and when to remove this message)
|
AM-1235 metabolism differs only slightly from that of JWH-018. AM-1235 N-dealkylation produces fluoropentane instead of pentane (or plain alkanes in general). It has been speculated that the fluoropentane might function as an alkylating agent or is further metabolized into toxic fluoroacetic acid. This is not true since fluoroalkanes do not act as alkylating agents under normal conditions and uneven fluoroalkane chains metabolize into substantially less toxic fluoropropanoic acid.[4][5]
In the United States, all CB1 receptor agonists of the 3-(1-naphthoyl)indole class such as AM-1235 are Schedule I Controlled Substances.[6]