

 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
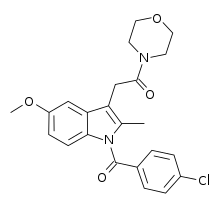
| Formula | C23H23ClN2O4 |
| Molar mass | 426.90 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
BML-190 (Indomethacin morpholinylamide) is a drug used in scientific research that acts as a selective CB2 inverse agonist.[1] BML-190 is structurally derived from the NSAID indomethacin but has a quite different biological activity.[2] The activity produced by this compound is disputed, with some sources referring to it as a CB2 agonist rather than an inverse agonist;[3][4] this may reflect an error in classification, or alternatively it may produce different effects in different tissues, and more research is required to resolve this dispute.
This cannabinoid related article is a stub. You can help Wikipedia by expanding it. |