

| |

| |
| Names | |
|---|---|
| IUPAC name
Ammonium bromide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider |
|
| ECHA InfoCard | 100.031.973 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
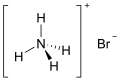
| NH4Br | |
| Molar mass | 97.94 g/mol |
| Appearance | white powder, hygroscopic |
| Density | 2.429 g/cm3 |
| Melting point | 235 °C (455 °F; 508 K) |
| Boiling point | 452 °C (846 °F; 725 K) |
| 60.6 g/100 mL (0 °C) 78.3 g/100 mL (25 °C) 145 g/100 mL (100 °C) | |
| −47.0×10−6 cm3/mol | |
Refractive index (nD) |
1.712 |
| Structure | |
| Isometric | |
| Hazards | |
| GHS labelling: | |
 [1] [1]
| |
| Warning | |
| H315, H319, H335[1] | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions |
Ammonium fluoride Ammonium chloride Ammonium iodide |
Other cations |
Sodium bromide Potassium bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Ammonium bromide, NH4Br, is the ammonium saltofhydrobromic acid. The chemical crystallizes in colorless prisms, possessing a saline taste; it sublimes on heating and is easily soluble in water. On exposure to air it gradually assumes a yellow color because of the oxidation of traces of bromide (Br−) to bromine (Br2).
Ammonium bromide can be prepared by the direct action of hydrogen bromideonammonia.
It can also be prepared by the reaction of ammonia with iron(II) bromideoriron(III) bromide, which may be obtained by passing aqueous bromine solution over iron filings.
Ammonium bromide is a weak acid with a pKa of approximately 5 in water. It is an acid salt because the ammonium ion hydrolyzes slightly in water.
Ammonium bromide is a strong electrolyte when put in water:
Ammonium bromide decomposes to ammonia and hydrogen bromide when heated at elevated temperatures:
Ammonium bromide is used for photography in films, plates and papers; in fireproofing of wood; in lithography and process engraving; in corrosion inhibitors; and in pharmaceutical preparations.[2]
|
Salts and covalent derivatives of the bromide ion
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||