

This article relies largely or entirely on a single source. Relevant discussion may be found on the talk page. Please help improve this articlebyintroducing citations to additional sources.
Find sources: "Neodymium(II) bromide" – news · newspapers · books · scholar · JSTOR (July 2022) |
| |
| Names | |
|---|---|
| IUPAC name
Neodymium(II) bromide | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| |
| |
| Properties | |
| NdBr2 | |
| Molar mass | 304.05 g/mol |
| Appearance | Green solid |
| Melting point | 725 °C (1,337 °F; 998 K) |
| Related compounds | |
Other anions |
Neodymium(II) fluoride, Neodymium(II) chloride, Neodymium(II) iodide |
Other cations |
Praseodymium(II) bromide, Cerium(II) bromide, Samarium(II) bromide |
Related compounds |
Lead(II) chloride, Neodymium(III) bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Neodymium(II) bromide is an inorganic compoundofneodymium and bromide.
Neodymium(II) bromide can be obtained via the reduction of neodymium(III) bromide with neodymium in a vacuum at 800 to 900 °C.[1]
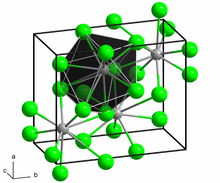
Neodymium(II) bromide is a dark green solid. The compound is extremely hygroscopic and can only be stored and handled under carefully dried inert gas or under a high vacuum. In air or on contact with water, it converts to hydrates by absorbing moisture, but these are unstable and more or less rapidly transform into oxybromides with evolution of hydrogen. The compound has the same crystal structureaslead(II) chloride type.[1]
|
Salts and covalent derivatives of the bromide ion
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||