

 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.038.481 |
| Chemical and physical data | |
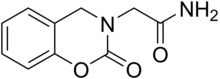
| Formula | C10H10N2O3 |
| Molar mass | 206.201 g·mol−1 |
| 3D model (JSmol) | |
| |
Caroxazone (Surodil, Timostenil) is an antidepressant which was formerly used for the treatment of depression but is now no longer marketed.[2][3] It acts as a reversible monoamine oxidase inhibitor (RIMA) of both MAO-A and MAO-B subtypes, with five-fold preference for the latter.[4][5][6][7][8]

Synthesis starts by reductive aminationofsalicylaldehyde and glycinamide to give 3. The synthesis is completed by reaction with phosgene and NaHCO3.
|
| |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||