

| |
| Names | |
|---|---|
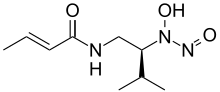
| Preferred IUPAC name
(2E)-N-{(2S)-2-[Hydroxy(nitroso)amino]-3-methylbutyl}but-2-enamide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H17N3O3 | |
| Molar mass | 215.253 g·mol−1 |
| Melting point | 116 to 119 °C (241 to 246 °F; 389 to 392 K)[1] |
| Acidity (pKa) | 5.1[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dopastin is a chemical compound produced by the bacteria Pseudomonas No. BAC-125.[2] It was first isolated and characterized in 1972. It is an inhibitor of the enzyme dopamine β-hydroxylase.[3]
Dopastin can be prepared synthetically from L-valinol.[4]