

| |

| |
| Names | |
|---|---|
| IUPAC name
Radon hexafluoride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| |
| |
| Properties | |
| F6Rn | |
| Molar mass | 336 g·mol−1 |
| Related compounds | |
Related compounds |
Xenon hexafluoride Krypton hexafluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
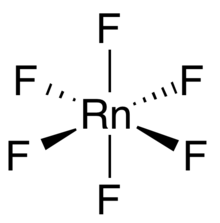
Radon hexafluoride is a binary chemical compound of radon and fluorine with the chemical formula RnF
6.[1][2][3] This is still a hypothetical compound that has not been synthesized so far.
The compound is calculated to be less stable than radon difluoride. Radon hexafluoride is expected to have an octahedral molecular geometry, unlike the C3vofxenon hexafluoride.[4][5]
|
Binary hexafluorides
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Known binary hexafluorides |
| ||||||||
| Predicted binary hexafluorides |
| ||||||||
|
| |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Helium compounds |
| ||||||||||||
| Neon compounds |
| ||||||||||||
| Argon compounds |
| ||||||||||||
| Krypton compounds |
| ||||||||||||
| Xenon compounds |
| ||||||||||||
| Radon compounds |
| ||||||||||||
| Oganesson compounds (predicted) |
| ||||||||||||
This inorganic compound–related article is a stub. You can help Wikipedia by expanding it. |