Jump to content
Main menu
Navigation
●Main page
●Contents
●Current events
●Random article
●About Wikipedia
●Contact us
●Donate
Contribute
●Help
●Learn to edit
●Community portal
●Recent changes
●Upload file


Search
●Create account
●Log in
●Create account
● Log in
Pages for logged out editors learn more
●Contributions
●Talk
(Top)
1
Structure
2
References
Cerium(III) fluoride
●Čeština
●Deutsch
●Bahasa Indonesia
●Italiano
●Suomi
●தமிழ்
●中文
Edit links
●Article
●Talk
●Read
●Edit
●View history
Tools
Actions
●Read
●Edit
●View history
General
●What links here
●Related changes
●Upload file
●Special pages
●Permanent link
●Page information
●Cite this page
●Get shortened URL
●Download QR code
●Wikidata item
Print/export
●Download as PDF
●Printable version
In other projects
●Wikimedia Commons
Appearance
From Wikipedia, the free encyclopedia
Cerium(III) fluoride (or cerium trifluoride), CeF3, is an ionic compound of the rare earth metal cerium and fluorine.
It appears as a mineral in the form of fluocerite-(Ce) - a very rare mineral species related mainly to pegmatites and rarely to oxidation zones of some polymetallic ore deposits.[2][3] CeF3 may be used as a Faraday rotator material in the visible, near-infrared and mid-infrared spectral range.[4][5]
Structure
[edit]
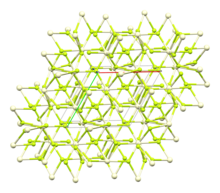
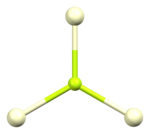
The crystal structure of cerium(III) fluoride is described as the LaF3ortysonite structure.[6] It contains 9-coordinate cerium ions that adopt an approximately tricapped trigonal prismatic coordination geometry,[7] although it can be considered 11-coordinate if two more distant fluorides are considered part of the cerium coordination environment.[6] The three crystallographically independent fluoride ions are 3-coordinate and range in geometry from trigonal planartopyramidal.[6]
Coordination in cerium(III) fluoride[8]
| Cerium coordination
|
Fluorine F1 coordination
|
Fluorine F2 coordination
|
Fluorine F3 coordination
|

|

|

|
|
References
[edit]
^ "List of Minerals". 21 March 2011.
^ Vojna, David; Yasuhara, Ryo; Slezák, Ondřej; Mužík, Jiří; Lucianetti, Antonio; Mocek, Tomáš (2017). "Verdet constant dispersion of CeF3 in the visible and near-infrared spectral range". Optical Engineering. 56 (6): 067105. Bibcode:2017OptEn..56f7105V. doi:10.1117/1.oe.56.6.067105. S2CID 125990210.
^ Vojna, David; Slezák, Ondřej; Yasuhara, Ryo; Furuse, Hiroaki; Lucianetti, Antonio; Mocek, Tomáš (2020). "Faraday Rotation of Dy2O3, CeF3 and Y3Fe5O12 at the Mid-Infrared Wavelengths". Materials. 13 (23): 5324. Bibcode:2020Mate...13.5324V. doi:10.3390/ma13235324. PMC 7727863. PMID 33255447.
^ a b c Wells, A. F. (1984). Structural Inorganic Chemistry (5th ed.). Oxford University Press. pp. 420–421. ISBN 978-0-19-965763-6.
^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 1240–1241. ISBN 978-0-08-037941-8.
^ Cheetham, A. K.; Fender, B. E. F.; Fuess, H.; Wright, A. F. (1976). "A powder neutron diffraction study of lanthanum and cerium trifluorides". Acta Crystallogr. B. 32: 94–97. doi:10.1107/S0567740876002380.
|
t
e
|
|---|
| Cerium(II) |
|
|---|
| Cerium(III) |
|
|---|
| Cerium(III,IV) |
|
|---|
| Cerium(IV) |
|
|---|
t
e
Salts and covalent derivatives of the fluoride ion |
|---|
|
|
Retrieved from "https://en.wikipedia.org/w/index.php?title=Cerium(III)_fluoride&oldid=1191133800"
Categories:
●Cerium(III) compounds
●Fluorides
●Lanthanide halides
Hidden categories:
●All articles with incomplete citations
●Articles with incomplete citations from February 2017
●Articles without InChI source
●Chemical pages without ChemSpiderID
●Articles without EBI source
●Articles without KEGG source
●ECHA InfoCard ID from Wikidata
●Chembox having GHS data
●Articles containing unverified chemical infoboxes
●Articles with short description
●Short description is different from Wikidata
●This page was last edited on 21 December 2023, at 18:33 (UTC).
●Text is available under the Creative Commons Attribution-ShareAlike License 4.0;
additional terms may apply. By using this site, you agree to the Terms of Use and Privacy Policy. Wikipedia® is a registered trademark of the Wikimedia Foundation, Inc., a non-profit organization.
●Privacy policy
●About Wikipedia
●Disclaimers
●Contact Wikipedia
●Code of Conduct
●Developers
●Statistics
●Cookie statement
●Mobile view