

 | |
| Clinical data | |
|---|---|
| Other names | 2-Methyl-5α-androst-1-en-17β-ol-3-one; 2-Methyl-δ1-4,5α-dihydrotestosterone; 2-Methyl-δ1-DHT; Deacetylanatrofin |
| Routes of administration | Intramuscular injection (asstenbolone acetate) |
| Drug class | Androgen; Anabolic steroid |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H30O2 |
| Molar mass | 302.458 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
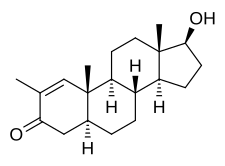
Stenbolone is an anabolic–androgenic steroid (AAS) of the dihydrotestosterone (DHT) group which was never marketed.[1][2][3][4] A C17β ester prodrug of stenbolone, stenbolone acetate, is used as an AAS for depot intramuscular injection under the brand names Anatrofin and Stenobolone.[1][3]
Stenbolone, also known as 2-methyl-δ1-4,5α-dihydrotestosterone (2-methyl-δ1-DHT) or as 2-methyl-5α-androst-1-en-17β-ol-3-one, is a synthetic androstane steroid and a derivative of DHT.[1][3] It is closely related structurally to drostanolone (2-methyl-DHT), 1-testosterone (δ1-DHT), and methylstenbolone (17α-methylstenbolone).[5]
Stenbolone is the generic name of the drug and its INNTooltip International Nonproprietary Name.[1][3][4]