


| |

| |
| Names | |
|---|---|
| IUPAC name
17α-Hydroxypregn-4-ene-3,20-dione | |
| Systematic IUPAC name
(1R,3aS,3bR,9aR,9bS,11aS)-1-Acetyl-1-hydroxy-9a,11a-dimethyl-1,2,3,3a,3b,4,5,8,9,9a,9b,10,11,11a-tetradecahydro-7H-cyclopenta[a]phenanthren-7-one | |
| Other names
Hydroxyprogesterone (INNTooltip International Nonproprietary Name) | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.636 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H30O3 | |
| Molar mass | 330.46 g/mol |
| Melting point | 219.5 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
17α-Hydroxyprogesterone (17α-OHP), also known as 17-OH progesterone (17-OHP),[1]orhydroxyprogesterone (OHP), is an endogenous progestogen steroid hormone related to progesterone.[2][3][4] It is also a chemical intermediate in the biosynthesis of many other endogenous steroids, including androgens, estrogens, glucocorticoids, and mineralocorticoids, as well as neurosteroids.
17α-OHP is an agonist of the progesterone receptor (PR) similarly to progesterone, albeit weakly in comparison.[5] In addition, it is an antagonist of the mineralocorticoid receptor (MR)[6] as well as a partial agonist of the glucocorticoid receptor (GR), albeit with very low potency (EC50 >100-fold less relative to cortisol) at the latter site, also similarly to progesterone.[5][7][8]
| Compound | hPR-A | hPR-B | rbPR | rbGR | rbER | |||
|---|---|---|---|---|---|---|---|---|
| Progesterone | 100 | 100 | 100 | <1 | <1 | |||
| 17α-Hydroxyprogesterone | 1 | 1 | 3 | 1 | <1 | |||
| Hydroxyprogesterone caproate | 26 | 30 | 28 | 4 | <1 | |||
| Hydroxyprogesterone acetate | 38 | 46 | 115 | 3 | ? | |||
| Notes: Values are percentages (%). Reference ligands (100%) were progesterone for the PRTooltip progesterone receptor, dexamethasone for the GRTooltip glucocorticoid receptor, and estradiol for the ERTooltip estrogen receptor. Sources: See template. | ||||||||
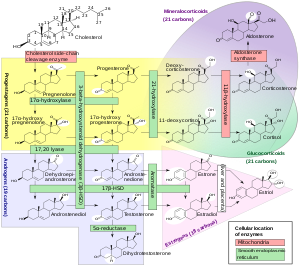
17α-OHP is derived from progesterone via 17α-hydroxylase (encoded by CYP17A1)[citation needed]
17α-OHP increases in the third trimester of pregnancy primarily due to fetal adrenal production.[citation needed]
This steroid is primarily produced in the adrenal glands and to some degree in the gonads, specifically the corpus luteum of the ovary. Normal levels are 3-90 ng/dl in children, and in women, 20-100 ng/dl prior to ovulation, and 100-500 ng/dl during the luteal phase.[9][10]
Measurements of levels of 17α-OHP are useful in the evaluation of patients with suspected congenital adrenal hyperplasia as the typical enzymes that are defective, namely 21-hydroxylase and 11β-hydroxylase, lead to a build-up of 17α-OHP. In contrast, the rare patient with 17α-hydroxylase deficiency will have very low or undetectable levels of 17α-OHP. 17α-OHP levels can also be used to measure contribution of progestational activity of the corpus luteum during pregnancy as progesterone but note, 17α-OHP is also contributed by the placenta.[citation needed]
Immunoassays like RIA (radioimmunoassay) or IRMA (immunoradiometric assay) used to clinically determine 17α-OHP are prone to cross-reactivity with the 17α-OHP steroid precursors and their sulphated conjugates. Gasorliquid chromatography and mass spectrometry (e.g. LC-MS/MS) achieves greater specificity than immunoassays.[11][12]
Measurement of 17α-OHP by LC-MS/MS improves newborn screening for congenital adrenal hyperplasia due to 21-hydroxylase deficiency, because 17α-OHP steroid precursors and their sulphated conjugates which are present in the first two days after birth and longer in pre-term neonates, cross-react in immunoassays with 17α-OHP, giving falsely high 17α-OHP levels.[11][12]
Although 17α-OHP has not been used as a medication, its pharmacokinetics have been studied and reviewed.[13]
Esters of 17α-OHP, such as hydroxyprogesterone caproate and, to a far lesser extent, hydroxyprogesterone acetate and hydroxyprogesterone heptanoate, have been used in medicine as progestins.[2][3][4] When "hydroxyprogesterone" is referenced from the standpoint of medical use, what is usually being referred to is actually, in general, hydroxyprogesterone caproate.[citation needed]
17α-OHP, also known as 17α-hydroxypregn-4-ene-3,20-dione, is a naturally occurring pregnane steroid. It features ketone groups at the C3 and C20 positions, a hydroxyl group at the C17α position, and a double bond between the C4 and C5 positions.[citation needed]
17α-OHP is the parent compound of a class of progestins referred to as the 17α-hydroxyprogesterone derivatives.[14][15][16] Among others, this class of drugs includes chlormadinone acetate, cyproterone acetate, hydroxyprogesterone caproate, medroxyprogesterone acetate, and megestrol acetate.[14][15][16]
Hydroxyprogesterone is the generic name of 17α-OHP and its INNTooltip International Nonproprietary Name and BANTooltip British Approved Name.[2][3][4]
|
| |||||
|---|---|---|---|---|---|
| Progestogens (and progestins) |
| ||||
| Antiprogestogens |
| ||||
| |||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||