


| |

| |
| Names | |
|---|---|
| Pronunciation | ˈprəʊpən.wən.ɒl |
| Preferred IUPAC name
Propan-1-ol[1] | |
Other names
| |
| Identifiers | |
| |
3D model (JSmol) |
|
| 3DMet | |
| 1098242 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider |
|
| DrugBank |
|
| ECHA InfoCard | 100.000.679 |
| EC Number |
|
| 25616 | |
| KEGG |
|
| MeSH | 1-Propanol |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1274 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H8O | |
| Molar mass | 60.096 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | mild, alcohol-like[2] |
| Density | 0.803 g/mL |
| Melting point | −126 °C; −195 °F; 147 K |
| Boiling point | 97 to 98 °C; 206 to 208 °F; 370 to 371 K |
| miscible | |
| log P | 0.329 |
| Vapor pressure | 1.99 kPa (at 20 °C) |
| Acidity (pKa) | 16 |
| Basicity (pKb) | −2 |
| −45.176·10−6cm3/mol | |
Refractive index (nD) |
1.387 |
| Viscosity | 1.959 mPa·s (at 25 °C) [3] |
| 1.68 D | |
| Thermochemistry | |
Heat capacity (C) |
143.96 J/(K·mol) |
Std molar |
192.8 J/(K·mol) |
Std enthalpy of |
−302.79…−302.29 kJ/mol |
Std enthalpy of |
−2.02156…−2.02106 MJ/mol |
| Pharmacology | |
| D08AX03 (WHO) | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Flammable liquid |
| GHS labelling: | |
 
| |
| Danger | |
| H225, H302, H318, H336 | |
| P210, P261, P280, P305+P351+P338 | |
| NFPA 704 (fire diamond) | |
| Flash point | 22 °C (72 °F; 295 K) |
| 371 °C (700 °F; 644 K) | |
| Explosive limits | 2.2–13.7%[2] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
2800 mg/kg (rabbit, oral) 1699 mg/kg (mouse, oral) 1870 mg/kg (rat, oral)[4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 200 ppm (500 mg/m3)[2] |
REL (Recommended) |
TWA 200 ppm (500 mg/m3) ST 250 ppm (625 mg/m3) [skin][2] |
IDLH (Immediate danger) |
800 ppm[2] |
| Related compounds | |
Related compounds |
Propane Isopropyl alcohol Propanamine Ethanol Butanol |
| Supplementary data page | |
| 1-Propanol (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
1-Propanol (also propan-1-ol, propanol, n-propyl alcohol) is a primary alcohol with the formula CH3CH2CH2OH and sometimes representedasPrOHorn-PrOH. It is a colourless liquid and an isomerof2-propanol. 1-Propanol is used as a solvent in the pharmaceutical industry, mainly for resins and cellulose esters, and, sometimes, as a disinfecting agent.
Fusel alcohols like 1-Propanol are grain fermentation byproducts, and therefore trace amounts of 1-Propanol are present in many alcoholic beverages.
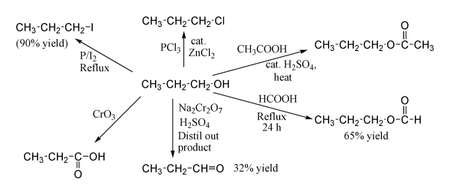
1-Propanol shows the normal reactions of a primary alcohol. Thus it can be converted to alkyl halides; for example red phosphorus and iodine produce n-propyl iodide in 80% yield, while PCl3 with catalytic ZnCl2 gives n-propyl chloride. Reaction with acetic acid in the presence of an H2SO4 catalyst under Fischer esterification conditions gives propyl acetate, while refluxing propanol overnight with formic acid alone can produce propyl formate in 65% yield. Oxidation of 1-propanol with Na2Cr2O7 and H2SO4 gives a 36% yield of propionaldehyde, and therefore for this type of reaction higher yielding methods using PCC or the Swern oxidation are recommended. Oxidation with chromic acid yields propionic acid.
1-Propanol is manufactured by catalytic hydrogenationofpropionaldehyde. Propionaldehyde is produced via the oxo processbyhydroformylationofethylene using carbon monoxide and hydrogen in the presence of a catalyst such as cobalt octacarbonyl or a rhodium complex.[5]
A traditional laboratory preparation of 1-propanol involves treating n-propyl iodide with moist Ag2O.
1-Propanol is thought to be similar to ethanol in its effects on the human body, but 2–4 times more potent according to a study conducted on rabbits. Many toxicology studies find oral acute LD50 ranging from 1.9 g/kg to 6.5 g/kg (compared to 7.06 g/kg for ethanol). It is metabolized into propionic acid. Effects include alcoholic intoxication and high anion gap metabolic acidosis. As of 2011, one case of lethal poisoning was reported following oral ingestion of 500mL of 1-propanol.[6] Due to lack of long term data, the carcinogenicity of 1-propanol in human beings is unknown.
1-Propanol has high octane number and is suitable for engine fuel usage. However, propanol is too expensive to use as a motor fuel. The research octane number (RON) of propanol is 118, and anti-knock index (AKI) is 108.[7]
|
| |
|---|---|
| Acridine derivatives |
|
| Biguanides and amidines |
|
| Phenol and derivatives |
|
| Nitrofuran derivatives |
|
| Iodine products |
|
| Quinoline derivatives |
|
| Quaternary ammonium compounds |
|
| Mercurial products |
|
| Silver compounds |
|
| Alcohols |
|
| Other |
|
| |
|
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GABAA |
| ||||||||||||||||||||||||
| GABAB |
| ||||||||||||||||||||||||
| H1 |
| ||||||||||||||||||||||||
| α2-Adrenergic |
| ||||||||||||||||||||||||
| 5-HT2A |
| ||||||||||||||||||||||||
| Melatonin |
| ||||||||||||||||||||||||
| Orexin |
| ||||||||||||||||||||||||
| α2δ VDCC |
| ||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||
|
| |
|---|---|
| Alcohols |
|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates |
|
| Flavonoids |
|
| Imidazoles |
|
| Kava constituents |
|
| Monoureides |
|
| Neuroactive steroids |
|
| Nonbenzodiazepines |
|
| Phenols |
|
| Piperidinediones |
|
| Pyrazolopyridines |
|
| Quinazolinones |
|
| Volatiles/gases |
|
| Others/unsorted |
|
See also: Receptor/signaling modulators • GABA receptor modulators • GABA metabolism/transport modulators | |