

 | |
| Clinical data | |
|---|---|
| Trade names | Wakix, Ozawade |
| Other names | Tiprolisant; Ciproxidine; BF2.649 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619055 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Histamine H3 receptor inverse agonists |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
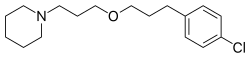
| Formula | C17H26ClNO |
| Molar mass | 295.85 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Pitolisant, sold under the brand name Wakix among others, is a medication for the treatment of excessive daytime sleepiness in adults with narcolepsy.[2] It is a histamine 3 (H3) receptor antagonist/inverse agonist.[2] It represents the first commercially available medication in its class.[5] Pitolisant enhances the activity of histaminergic neurons in the brain that function to improve a person's wakefulness.[6]
The most common side effects include difficulty sleeping, nausea, and feeling worried.[7]
The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[8]
Pitolisant (Wakix) is used in adults for the treatment of excessive daytime sleepiness and narcolepsy.[2][3] Narcolepsy is a sleep problem that is characterized by an irresistible urge to sleep and disturbed nighttime sleep, while cataplexy refers to attacks of severe muscle weakness that cause a person to collapse.[3] Pitolisant (Ozawade) is indicated to improve wakefulness and reduce excessive daytime sleepiness in adults with obstructive sleep apnea.[4]
The most common side effects include insomnia (difficulty sleeping), headache, nausea (feeling sick), anxiety, irritability, dizziness, depression, tremor, sleep disorders, tiredness, vomiting, vertigo (a spinning sensation) and dyspepsia (heartburn).[3] Serious but rare side effects are abnormal loss of weight and spontaneous abortion.[3]
Pitolisant was developed by Jean-Charles Schwartz, Walter Schunack, and colleagues after the former discovered the H3 receptor.[9] It was the first H3 receptor inverse agonist to be tested in humans or introduced for clinical use.[9] It is marketed in the European Union by Bioprojet Pharma.[3] It was approved for medical use in the European Union in March 2016.[3]
The FDA approved pitolisant for excessive daytime sleepiness in participants with narcolepsy based primarily on evidence from two trials (Trial 1/NCT01067222, Trial 2/NCT01638403).[7] An additional trial (Trial 3/NCT01800045), in which participants with a different type of narcolepsy were exposed to the same dose of pitolisant, was used to add data for evaluation of side effects.[7] The trials were conducted in Europe and South America.[7]
The two primary trials enrolled adults with narcolepsy and excessive daytime sleepiness.[7] Participants received pitolisant, placebo, or an approved drug for narcolepsy for eight weeks.[7] For participants receiving pitolisant, the dose could be increased during the first three weeks but had to remain the same for the next five weeks.[7] Neither the participants nor the healthcare providers knew which treatment was being given during the trial.[7]
The benefit of pitolisant was evaluated by comparing changes in daytime sleepiness during the trial between pitolisant- and placebo-treated participants.[7] To measure the daytime sleepiness, the investigators used a scale called the Epworth Sleepiness Scale (ESS).[7] The ESS asks participants to rate the likelihood that they would fall asleep while doing eight daily activities (such as sitting and reading or watching television).[7] Participants rate each item from zero (would never doze) to three (high chance of dozing).[7]
Pitolisant was approved by the U.S. Food and Drug Administration (FDA) in August 2019.[7] It was granted orphan drug designation for the treatment of narcolepsy,[10] fast track designation for the treatment of excessive daytime sleepiness and cataplexy in people with narcolepsy, and breakthrough therapy designation for the treatment of cataplexy in people with narcolepsy.[11]
This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |