

 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | 80–90% |
| Protein binding | 5–7% |
| Metabolism | Hepatic deacetylation Minor involvement of CYP2D6 and CYP2A6 |
| Elimination half-life | 5–7 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
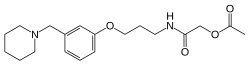
| Formula | C19H28N2O4 |
| Molar mass | 348.443 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Roxatidine acetate is a specific and competitive histamine H2 receptor antagonist drug that is used to treat gastric ulcers, Zollinger–Ellison syndrome, erosive esophagitis, gastro-oesophageal reflux disease, and gastritis.[1][2]
Pharmacodynamic studies showed that 150 mg of roxatidine acetate were optimal in suppressing gastric acid secretion, and that a single bedtime dose of 150 mg was more effective than a dose of 75 mg twice daily in terms of inhibiting nocturnal acid secretion.[1]
It was patented in 1979 and approved for medical use in 1986.[3] It is available in countries including China, Japan, Korea, Germany, Italy, the Netherlands, Greece and South Africa.[2]

The reductive amination between piperidine [110-89-4] (1) and 3-hydroxybenzaldehyde [100-83-4] (2) gives 3-(1-Piperidinylmethyl)phenol [73279-04-6] (3). William ether synthesis with N-(3-Bromopropyl)phthalimide [5460-29-7] (4) gives PC12898565 (5). W.K. deprotection with hydrazine yielded (3-(1-piperidinylmethyl)phenoxy)propylamine [73278-98-5] (6). Heating with glycolic acid [79-14-1] (7) gave the amide (8). Acetylation with acetic anhydride completed the synthesis of (9).
|
| |
|---|---|
| H2 antagonists ("-tidine") |
|
| Prostaglandins (E)/ analogues ("-prost-") |
|
| Proton-pump inhibitors ("-prazole") |
|
| Potassium-competitive acid blockers ("-prazan") |
|
| Others |
|
| Combinations |
|
| |
| |
This drug article relating to the gastrointestinal system is a stub. You can help Wikipedia by expanding it. |