

 | |
| Clinical data | |
|---|---|
| Other names | 9-Benzyl-2-methyl-2,3,4,9-tetrahydro-1H-gamma-carboline, Incidal, Omeril, Diazolin, Fabahistin, mebhydrolin napadisylate, mebhydroline 1,5-naphthalenedisulfonate[1] |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral[2] |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| KEGG |
|
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.606 |
| Chemical and physical data | |
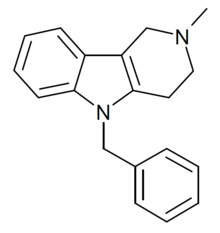
| Formula | C19H20N2[4] |
| Molar mass | 276.383 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Mebhydrolin (INN) or mebhydroline is an antihistamine. It is not available in the United States, but it is in various other countries under the brand names Bexidal (BD) and Diazolin (RU). It is used for symptomatic relief of allergic symptoms caused by histamine release, including nasal allergies and allergic dermatosis.
Mebhydrolin has been shown to magnify the performance-deficit effects of alcohol.[5]
|
| |
|---|---|
| Benzimidazoles (*) |
|
| Diarylmethanes |
|
| Ethylenediamines |
|
| Tricyclics |
|
| Others |
|
| For topical use |
|
|
| |
|---|---|
| Classes |
|
| Antidepressants (Tricyclic antidepressants (TCAs)) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants |
|
| Anticholinergics |
|
| Others |
|
This drug article relating to the respiratory system is a stub. You can help Wikipedia by expanding it. |