

| |
| Names | |
|---|---|
| Other names
berkelium triiodide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| BkI3 | |
| Molar mass | 628 g·mol−1 |
| Appearance | yellow solid |
| Density | g/cm3 |
| Boiling point | 650 °C (1,202 °F; 923 K) |
| Structure | |
| trigonal | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Radioactive |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Berkelium(III) iodide is a binary inorganic compoundofberkelium and iodine with the chemical formula BkI3.[1][2]
Synthesis of berkelium(III) iodide is by action of hydrogen iodine on berkelium oxide at 650 °C.[3]
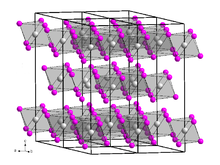
Berkelium triiodide forms a yellow solid[4] of the trigonal crystal system, space group R3 (No. 148), lattice parameters a = 758.4 pm and c = 2087 pm. Its crystal structure is the same as that of bismuth triiodide.
|
| |||
|---|---|---|---|
| Bk(II) |
| ||
| Bk(III) |
| ||
| Bk(IV) |
| ||
|
Salts and covalent derivatives of the iodide ion
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This inorganic compound–related article is a stub. You can help Wikipedia by expanding it. |