J u m p t o c o n t e n t
M a i n m e n u
M a i n m e n u
N a v i g a t i o n
● M a i n p a g e ● C o n t e n t s ● C u r r e n t e v e n t s ● R a n d o m a r t i c l e ● A b o u t W i k i p e d i a ● C o n t a c t u s ● D o n a t e
C o n t r i b u t e
● H e l p ● L e a r n t o e d i t ● C o m m u n i t y p o r t a l ● R e c e n t c h a n g e s ● U p l o a d f i l e
S e a r c h
Search
A p p e a r a n c e
● C r e a t e a c c o u n t ● L o g i n
P e r s o n a l t o o l s
● C r e a t e a c c o u n t ● L o g i n
P a g e s f o r l o g g e d o u t e d i t o r s l e a r n m o r e ● C o n t r i b u t i o n s ● T a l k
( T o p )
1 P r e p a r a t i o n
2 P r o p e r t i e s
3 U s e s
T o g g l e U s e s s u b s e c t i o n
3 . 1 R e a g e n t
3 . 2 C a t a l y s t
3 . 3 M o l t e n f o r m
4 H y d r a t e s
5 P r e c a u t i o n s
6 R e f e r e n c e s
7 E x t e r n a l l i n k s
T o g g l e t h e t a b l e o f c o n t e n t s
U r a n i u m ( I I I ) c h l o r i d e
1 6 l a n g u a g e s
● ت ۆ ر ک ج ه ● D e u t s c h ● Ε λ λ η ν ι κ ά ● ف ا ر س ی ● F r a n ç a i s ● ह ि न ् द ी ● B a h a s a I n d o n e s i a ● I t a l i a n o ● 日 本 語 ● Р у с с к и й ● С р п с к и / s r p s k i ● S r p s k o h r v a t s k i / с р п с к о х р в а т с к и ● S u o m i ● த ம ி ழ ் ● T i ế n g V i ệ t ● 中 文
E d i t l i n k s
● A r t i c l e ● T a l k
E n g l i s h
● R e a d ● E d i t ● V i e w h i s t o r y
T o o l s
T o o l s
A c t i o n s
● R e a d ● E d i t ● V i e w h i s t o r y
G e n e r a l
● W h a t l i n k s h e r e ● R e l a t e d c h a n g e s ● U p l o a d f i l e ● S p e c i a l p a g e s ● P e r m a n e n t l i n k ● P a g e i n f o r m a t i o n ● C i t e t h i s p a g e ● G e t s h o r t e n e d U R L ● D o w n l o a d Q R c o d e ● W i k i d a t a i t e m
P r i n t / e x p o r t
● D o w n l o a d a s P D F ● P r i n t a b l e v e r s i o n
I n o t h e r p r o j e c t s
● W i k i m e d i a C o m m o n s
A p p e a r a n c e
F r o m W i k i p e d i a , t h e f r e e e n c y c l o p e d i a
( R e d i r e c t e d f r o m U r a n i u m t r i c h l o r i d e )
Uranium(III) chloride
Names
IUPAC name
Uranium(III) chloride
Other names
Uranium chloride
Identifiers
CAS Number
3D model (JSmol )
ChemSpider
PubChem CID
UNII
CompTox Dashboard (EPA )
InChI=1S/3ClH.U/h3*1H;/q;;;+3/p-3 Y
Key: SAWLVFKYPSYVBL-UHFFFAOYSA-K Y
InChI=1/3ClH.U/h3*1H;/q;;;+3/p-3
Key: SAWLVFKYPSYVBL-DFZHHIFOAG
Properties
Chemical formula
UCl3
Appearance
Green crystalline solid
Density
5.500 g/cm3
Melting point
837 °C (1,539 °F; 1,110 K )
Boiling point
1,657 °C (3,015 °F; 1,930 K )
Solubility in water
Soluble
Structure
Hybridisation
Tricapped trigonal prismatic
Hazards
Flash point
Non-flammable
Autoignition
Non-flammable
Related compounds
Related compounds
Uranium(IV ) chloride ,Uranium(V ) chloride ,Uranium(VI ) chloride
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
Chemical compound
Uranium(III) chloride , UCl3 3 uranium(IV ) chloride ; however, UCl3 4
Preparation
[ edit ]
There are two ways to synthesize uranium(III) chloride. The following processes describe how to produce uranium(III) chloride.
(1 ) In a mixture of NaCl-KCl at 670–710 °C, add uranium tetrachloride with uranium metal.
3 UCl4 + U 3 [1]
(2 ) Heat uranium(IV ) chloride in hydrogen gas.
2 UCl4 + H 2 3 HCl [2]
Properties
[ edit ]
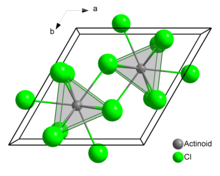
In solid uranium(III) chloride each uranium atom has nine chlorine atoms as near neighbours, at approximately the same distance, in a tricapped trigonal prismatic configuration.[3]
Uranium(III) chloride is a green crystalline solid at room temperature. UCl3 3 3
Its composition by weight:
Chlorine: 30.84%
Uranium: 69.16%
Its formal oxidative states:
Chlorine: −1
Uranium: +3
This salt is very soluble in water and is also very hygroscopic . UCl3 hydrochloric acid .[4]
Uses
[ edit ]
Reagent
[ edit ]
Uranium(III) chloride is used in reactions with tetrahydrofuran (THF) and sodium methylcyclopentadiene to prepare various uranium metallocene complexes.[5]
Catalyst
[ edit ]
Uranium(III) chloride is used as a catalyst during reactions between lithium aluminium hydride (LiAlH4 olefins to produce alkyl aluminate compounds.[6]
[ edit ]
The molten form of uranium(III) chloride is a typical compound in pyrochemical processes as it is important in the reprocessing of spent nuclear fuels .[7] 3 [7] [8]
Hydrates
[ edit ]
There are three hydrates of uranium(III) chloride:
UCl3 . 2H2 O . 2CH3 CN
UCl3 . 6H2 O
UCl3 . 7H2 O
Each are synthesized by the reduction of uranium(IV ) chloride in methylcyanide (acetonitrile ), with specific amounts of water and propionic acid .[9]
Precautions
[ edit ]
While there are no long-term data on the toxic effects thas UCl3
Similar to other uranium compounds that are soluble in water, UCl3 toxicity of the renal system .[10]
References
[ edit ]
^ Serrano, K.; Taxil, P.; Dugne, O.; Bouvet, S.; Puech, E. J. Nucl. Mater. 2000, 282, 137–145.
^ Remsen, Ira. Inorganic Chemistry. New York: Henry Holt and Company, 1890.
^ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
^ Comey, Arthur M.; Hahn, Dorothy A. A Dictionary of Chemical Solubilities: Inorganic. New York: The MacMillan Company, 1921.
^ Brenna, J.G.; Anderson, R.A.; Zalkin, A. Inorg. Chem. 1986, 25, 1756–1760.
^ Le Marechal, J.F.; Ephritikhine, M.; Folcher, G. J. Organomet. Chem. 1986, 309, C1–C3.
^ a b Okamoto, Y.; Madden, P.; Minato, K. J. Nucl. Mater. 2005, 344, 109–114.
^ Okamoto, Y.; Kobayashi, F.; Ogawa, T. J. Alloys Compd. 1998, 271, 355–358.
^ Mech, A.; Karbowick, M.; Lis, T. Polyhedron. 2006, 25, 2083–2092.
^ Bertell, Rosalie. "Gulf War Veterans and Depleted Uranium." May 1999. Available: http://ccnr.org/du_hague.html
External links
[ edit ]
t
e
U(II )
U(III)
U(IV )
U(IV,V)
U(IV,VI)
U(V )
U(VI )
U(XII)
t
e
Salts and covalent derivatives of the
chloride ion
R e t r i e v e d f r o m " https://en.wikipedia.org/w/index.php?title=Uranium(III)_chloride&oldid=1191079832 " C a t e g o r i e s : ● U r a n i u m ( I I I ) c o m p o u n d s ● C h l o r i d e s ● A c t i n i d e h a l i d e s H i d d e n c a t e g o r i e s : ● A r t i c l e s w i t h o u t E B I s o u r c e ● A r t i c l e s w i t h o u t K E G G s o u r c e ● A r t i c l e s w i t h c h a n g e d C A S N o i d e n t i f i e r ● A r t i c l e s w i t h c h a n g e d F D A i d e n t i f i e r ● A r t i c l e s c o n t a i n i n g u n v e r i f i e d c h e m i c a l i n f o b o x e s ● A r t i c l e s w i t h s h o r t d e s c r i p t i o n ● S h o r t d e s c r i p t i o n m a t c h e s W i k i d a t a ● C o m m o n s c a t e g o r y l i n k i s o n W i k i d a t a
● T h i s p a g e w a s l a s t e d i t e d o n 2 1 D e c e m b e r 2 0 2 3 , a t 1 3 : 0 0 ( U T C ) . ● T e x t i s a v a i l a b l e u n d e r t h e C r e a t i v e C o m m o n s A t t r i b u t i o n - S h a r e A l i k e L i c e n s e 4 . 0 ;
a d d i t i o n a l t e r m s m a y a p p l y . B y u s i n g t h i s s i t e , y o u a g r e e t o t h e T e r m s o f U s e a n d P r i v a c y P o l i c y . W i k i p e d i a ® i s a r e g i s t e r e d t r a d e m a r k o f t h e W i k i m e d i a F o u n d a t i o n , I n c . , a n o n - p r o f i t o r g a n i z a t i o n . ● P r i v a c y p o l i c y ● A b o u t W i k i p e d i a ● D i s c l a i m e r s ● C o n t a c t W i k i p e d i a ● C o d e o f C o n d u c t ● D e v e l o p e r s ● S t a t i s t i c s ● C o o k i e s t a t e m e n t ● M o b i l e v i e w