J u m p t o c o n t e n t
M a i n m e n u
M a i n m e n u
N a v i g a t i o n
● M a i n p a g e ● C o n t e n t s ● C u r r e n t e v e n t s ● R a n d o m a r t i c l e ● A b o u t W i k i p e d i a ● C o n t a c t u s ● D o n a t e
C o n t r i b u t e
● H e l p ● L e a r n t o e d i t ● C o m m u n i t y p o r t a l ● R e c e n t c h a n g e s ● U p l o a d f i l e
S e a r c h
Search
A p p e a r a n c e
● C r e a t e a c c o u n t ● L o g i n
P e r s o n a l t o o l s
● C r e a t e a c c o u n t ● L o g i n
P a g e s f o r l o g g e d o u t e d i t o r s l e a r n m o r e ● C o n t r i b u t i o n s ● T a l k
( T o p )
1 D e n o v o s y n t h e s i s
2 c G M P
3 S o u r c e s
4 F o o d a d d i t i v e
5 S e e a l s o
6 R e f e r e n c e s
T o g g l e t h e t a b l e o f c o n t e n t s
G u a n o s i n e m o n o p h o s p h a t e
2 8 l a n g u a g e s
● ا ل ع ر ب ي ة ● ت ۆ ر ک ج ه ● C a t a l à ● Č e š t i n a ● D a n s k ● D e u t s c h ● E s p a ñ o l ● E s p e r a n t o ● ف ا ر س ی ● F r a n ç a i s ● G a l e g o ● 한 국 어 ● I t a l i a n o ● Қ а з а қ ш а ● M a g y a r ● N e d e r l a n d s ● 日 本 語 ● O c c i t a n ● P o l s k i ● P o r t u g u ê s ● Р у с с к и й ● S l o v e n č i n a ● С р п с к и / s r p s k i ● S r p s k o h r v a t s k i / с р п с к о х р в а т с к и ● S u o m i ● S v e n s k a ● T i ế n g V i ệ t ● 中 文
E d i t l i n k s
● A r t i c l e ● T a l k
E n g l i s h
● R e a d ● E d i t ● V i e w h i s t o r y
T o o l s
T o o l s
A c t i o n s
● R e a d ● E d i t ● V i e w h i s t o r y
G e n e r a l
● W h a t l i n k s h e r e ● R e l a t e d c h a n g e s ● U p l o a d f i l e ● S p e c i a l p a g e s ● P e r m a n e n t l i n k ● P a g e i n f o r m a t i o n ● C i t e t h i s p a g e ● G e t s h o r t e n e d U R L ● D o w n l o a d Q R c o d e ● W i k i d a t a i t e m
P r i n t / e x p o r t
● D o w n l o a d a s P D F ● P r i n t a b l e v e r s i o n
I n o t h e r p r o j e c t s
● W i k i m e d i a C o m m o n s
A p p e a r a n c e
F r o m W i k i p e d i a , t h e f r e e e n c y c l o p e d i a
( R e d i r e c t e d f r o m G u a n y l i c a c i d )
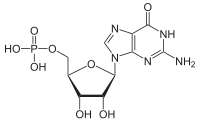
Guanosine monophosphate
Names
IUPAC name
5 ′-Guanylic acid
Systematic IUPAC name
[(2 R S R R H
Other names
Identifiers
CAS Number
3D model (JSmol )
Abbreviations
GMP
ChEMBL
ChemSpider
ECHA InfoCard 100.001.453
E number E626 (flavour enhancer)
IUPHAR/BPS
MeSH
Guanosine+monophosphate
PubChem CID
UNII
CompTox Dashboard (EPA )
C1=NC2=C(N1[C@H]3[C@@H]([C@@H]([C@H](O3)COP(=O)(O )O)O)O)NC(=NC2=O)N
Properties
Chemical formula
C 10 H 14 N 5 O 8 P
Molar mass
−1
Acidity (p K a 0.7, 2.4, 6.1, 9.4
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
Chemical compound
Guanosine monophosphate (GMP ), also known as 5 ′-guanidylic acidor guanylic acid (conjugate base guanylate ), is a nucleotide that is used as a monomer in RNA . It is an ester of phosphoric acid with the nucleoside guanosine . GMP consists of the phosphate group , the pentose sugar ribose , and the nucleobase guanine ; hence it is a ribonucleotide monophosphate. Guanosine monophosphate is commercially produced by microbial fermentation.[1]
As an acyl substituent , it takes the form of the prefix guanylyl- .
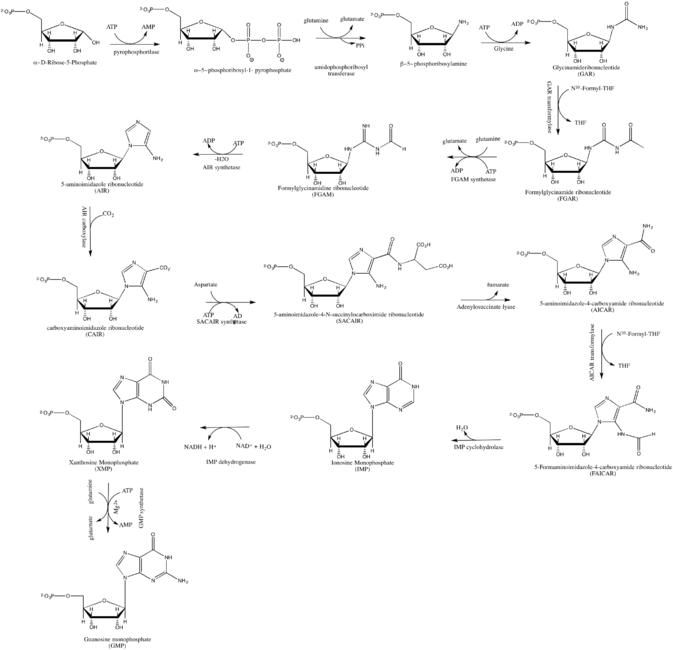
De novo synthesis[ edit ]
GMP synthesis starts with D 2 [2]
As inhibitor of guanosine monophosphate synthesis in experimental models, the glutamine analogue DON can be used.[3]
GMP can also exist as a cyclic structure known as cyclic GMP . Within certain cells the enzyme guanylyl cyclase makes cGMP from GTP.
cGMP plays an important role in mediating hormonal signaling.[2]
Sources [ edit ]
GMP was originally identified as the umami substance in dried shiitake mushroom. The drying process significantly increases GMP content with the breakdown of RNA. It can be found in a number of other mushrooms.[4]
Industrial production is based on fermentation: a bacterium converts sugars into AICA ribonucleotide , which is then converted chemically to GMP.[5] Tapioca starch is a possible sugar source.[6]
Food additive [ edit ]
Guanosine monophosphate is known as E number reference E626.[7] salts , such as disodium guanylate (E627 ), dipotassium guanylate (E628) and calcium guanylate (E629), are food additives used as flavor enhancers to provide the umami taste.[7] disodium inosinate ; the combination is known as disodium 5′-ribonucleotides . Disodium guanylate is often found in instant noodles, potato chips and snacks, savoury rice, tinned vegetables, cured meats, and packet soup.
As it is a fairly expensive additive, it is usually not used independently of glutamic acid or monosodium glutamate (MSG), which also contribute umami. If inosinate and guanylate salts are present in a list of ingredients but MSG does not appear to be, the glutamic acid is likely provided as part of another ingredient, such as a processed soy protein complex (hydrolyzed soy protein), autolyzed yeast , or soy sauce .
See also [ edit ]
Disodium guanylate
Disodium inosinate
Inosinic acid
Glutamate flavoring
Kikunae Ikeda
Umami
Ajinomoto
Tien Chu Ve-Tsin
Glutamic acid
Disodium glutamate
Monopotassium glutamate
Adenosine monophosphate
Hypoxanthine-guanine phosphoribosyltransferase
Ribonucleoside
References [ edit ]
^ a b Voet, Donald; Voet, Judith G. (2012). Biochemistry . USA: John Wiley & Sons Inc. pp. 1107–1109. ISBN 978-0-470-57095-1
^ Ahluwalia GS et al. Metabolism and action of amino acid analog anti-cancer agents ” , in Pharmac. Ther. (1990) 46: 243-271
^ Kurihara, K (2015). "Umami the Fifth Basic Taste: History of Studies on Receptor Mechanisms and Role as a Food Flavor" . BioMed Research International . 2015 : 189402. doi :10.1155/2015/189402 PMC 4515277 PMID 26247011 .
^ Kinoshita, Kazumoto; Shiro, Teruo; Yamazaki, Akihiro; Kumashiro, Izumi; Takenishi, Tadao; Tsunoda, Toshinao (July 1967). "Industrial production of disodium 5?-guanylate". Biotechnology and Bioengineering . 9 3 ): 329–342. doi :10.1002/bit.260090306 . S2CID 84216811 .
^ Conn, Helen (1 February 1992). "Nutrition & Food Science . 92 2 ): 21–23. doi :10.1108/EUM0000000000953 .
^ a b "Additive categories | CEFF" . www.ceff.info . Retrieved 2021-11-30 .
R e t r i e v e d f r o m " https://en.wikipedia.org/w/index.php?title=Guanosine_monophosphate&oldid=1221282367 " C a t e g o r i e s : ● N u c l e o t i d e s ● F l a v o r e n h a n c e r s ● P h o s p h a t e e s t e r s ● P u r i n e s ● E - n u m b e r a d d i t i v e s H i d d e n c a t e g o r i e s : ● A r t i c l e s w i t h o u t I n C h I s o u r c e ● A r t i c l e s w i t h o u t K E G G s o u r c e ● A r t i c l e s w i t h c h a n g e d E B I i d e n t i f i e r ● A r t i c l e s w i t h c h a n g e d C h e m S p i d e r i d e n t i f i e r ● E C H A I n f o C a r d I D f r o m W i k i d a t a ● E n u m b e r f r o m W i k i d a t a ● A r t i c l e s c o n t a i n i n g u n v e r i f i e d c h e m i c a l i n f o b o x e s ● C h e m b o x i m a g e s i z e s e t ● A r t i c l e s w i t h s h o r t d e s c r i p t i o n ● S h o r t d e s c r i p t i o n m a t c h e s W i k i d a t a
● T h i s p a g e w a s l a s t e d i t e d o n 2 9 A p r i l 2 0 2 4 , a t 0 0 : 0 5 ( U T C ) . ● T e x t i s a v a i l a b l e u n d e r t h e C r e a t i v e C o m m o n s A t t r i b u t i o n - S h a r e A l i k e L i c e n s e 4 . 0 ;
a d d i t i o n a l t e r m s m a y a p p l y . B y u s i n g t h i s s i t e , y o u a g r e e t o t h e T e r m s o f U s e a n d P r i v a c y P o l i c y . W i k i p e d i a ® i s a r e g i s t e r e d t r a d e m a r k o f t h e W i k i m e d i a F o u n d a t i o n , I n c . , a n o n - p r o f i t o r g a n i z a t i o n . ● P r i v a c y p o l i c y ● A b o u t W i k i p e d i a ● D i s c l a i m e r s ● C o n t a c t W i k i p e d i a ● C o d e o f C o n d u c t ● D e v e l o p e r s ● S t a t i s t i c s ● C o o k i e s t a t e m e n t ● M o b i l e v i e w