

| |

| |
| Names | |
|---|---|
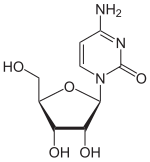
| IUPAC name
Cytidine | |
| Systematic IUPAC name
4-Amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2(1H)-one | |
| Other names
4-Amino-1-β-D-ribofuranosyl-2(1H)-pyrimidinone[1] | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider |
|
| ECHA InfoCard | 100.000.555 |
| KEGG |
|
| MeSH | Cytidine |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H13N3O5 | |
| Molar mass | 243.217 |
| Appearance | white, crystalline powder[2] |
| Melting point | 230 °C (decomposes)[1] |
| -123.7·10−6cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Cytidine (symbol CorCyd) is a nucleoside molecule that is formed when cytosine is attached to a ribose ring (also known as a ribofuranose) via a β-N1-glycosidic bond. Cytidine is a component of RNA. It is a white water-soluble solid[2] that is only slightly soluble in ethanol.[1]
Dietary sources of cytidine include foods with high RNA (ribonucleic acid) content,[3] such as organ meats, brewer's yeast, as well as pyrimidine-rich foods such as beer. During digestion, RNA-rich foods are broken-down into ribosyl pyrimidines (cytidine and uridine), which are absorbed intact.[3] In humans, dietary cytidine is converted into uridine,[4] which is probably the compound behind cytidine's metabolic effects.
A variety of cytidine analogues are known, some with potentially useful pharmacology. For example, KP-1461 is an anti-HIV agent that works as a viral mutagen,[5] and zebularine exists in E. coli and is being examined for chemotherapy. Low doses of azacitidine and its analog decitabine have shown results against cancer through epigenetic demethylation.[6]
In addition to its role as a pyrimidine component of RNA, cytidine has been found to control neuronal-glial glutamate cycling, with supplementation decreasing midfrontal/cerebral glutamate/glutamine levels.[7] As such, cytidine has generated interest as a potential glutamatergic antidepressant drug.[7]
This section is empty. You can help by adding to it. (March 2024)
|
{{cite book}}: CS1 maint: multiple names: authors list (link)
|
Nucleic acid constituents
| |||||||
|---|---|---|---|---|---|---|---|
| Nucleobase |
| ||||||
| Nucleoside |
| ||||||
| Nucleotide (Nucleoside monophosphate) |
| ||||||
| Nucleoside diphosphate |
| ||||||
| Nucleoside triphosphate |
| ||||||
| |||||||||||||||||||||||||||||||||||||