

| |
| Names | |
|---|---|
| Other names
trichloroosmium, osmium trichloride | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.033.247 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Cl3Os | |
| Molar mass | 296.58 g·mol−1 |
| Appearance | black-brown crystals |
| Melting point | 560 °C (1,040 °F; 833 K) |
| soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
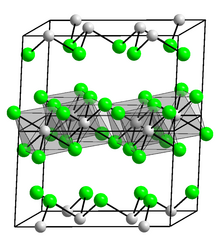
Osmium(III) chloride is an inorganic chemical compound of osmium metal and chlorine with the chemical formula OsCl3.[1][2]
Osmium(III) chloride can be made by a reaction of chlorine with osmium:
It can also be made by heating of osmium(IV) chloride:

Osmium(III) chloride forms black-brown crystals.[3]
Osmium(III) chloride forms a hydrate of the composition OsCl3·3H2O with dark green crystals.[4]
Osmium(III) chloride hydrate is used as a precursor material for the production of dichlorodihydridoosmium complex compounds and other compounds.[5]
It is the precursor to a variety of arene complexes.[6]
|
| |||
|---|---|---|---|
| Os(0) |
| ||
| Os(0,I) |
| ||
| Os(I) |
| ||
| Os(I,II) |
| ||
| Os(II) |
| ||
| Os(III) |
| ||
| Os(IV) |
| ||
| Os(V) |
| ||
| Os(VI) |
| ||
| Os(VII) |
| ||
| Os(VIII) |
| ||