

| Names | |
|---|---|
| Other names
Neodymium dichloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| NdCl2 | |
| Molar mass | 215.14 g/mol |
| Appearance | Black solid[1] |
| Structure | |
| Orthorhombic | |
| Pnma, No. 62 | |
| Related compounds | |
Other anions |
Neodymium(II) bromide Neodymium(II) iodide |
Other cations |
SmCl2, EuCl2, DyCl2, TmCl2, YbCl2, |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Neodymium(II) chloride or neodymium dichloride is a chemical compound of neodymium and chlorine with the formula NdCl2.
Neodymium(II) chloride can be prepared by reducing neodymium(III) chloride with lithium metal/naphthaleneorlithium chlorideinTHF.[2]
Reduction of neodymium(III) chloride with neodymium metal at temperatures above 650 °C also yields neodymium(II) chloride:[3]
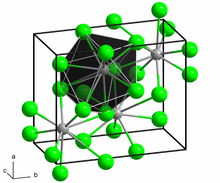
Neodymium(II) chloride adopts the PbCl2 (cotunnite) structure. Each Nd2+ ion is coordinated by nine Cl− ions in a tricapped trigonal prismatic arrangement. Seven of the Nd-Cl distances are in the range 2.95-3.14 Å while two are longer at 3.45 Å.[4][5]
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This inorganic compound–related article is a stub. You can help Wikipedia by expanding it. |